In non-rigid diatomic molecule with an additional vibrational mode
Important Questions on Kinetic Theory
Directions: Each of the following sentences in this section has a blank space and four words or group of words given after the sentence. Select the word or group of words you consider most appropriate for the blank space and indicate your response on the Answer Sheet accordingly.
Every rash driver becomes a ____ killer.
(R-Universal gas constant)
Given below are two statements:
Statement I: In a diatomic molecule, the rotational energy at a given temperature obeys Maxwell's distribution.
Statement II : In a diatomic molecule, the rotational energy at a given temperature equals the translational kinetic energy for each molecule.
In the light of the above statements, choose the correct answer from the options given below:
Two sentences are given below and you are required to find the correct sentence which combines both the sentences.
Which is the correct combination of the given two sentences?
He is too tired. He could not stand.
Match the ratio for ideal gases with different type of molecules:
| Molecule Type | |
| (A) Monoatomic | (I) |
| (B) Diatomic rigid molecules | (II) |
| (C) Diatomic non-rigid molecules | (III) |
| (D) Triatomic rigid molecules | (IV) |
In the following sentence, a blank space with four options is given. Select whichever preposition or determiner you consider the most appropriate for the blank space.
These are the good rules to live _____.
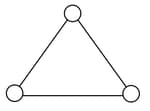
Consider a gas of triatomic molecules. The molecules are assumed to be triangular and made of massless rigid rods whose vertices are occupied by atoms. The internal energy of a mole of the gas at temperature T is:

