MEDIUM
Earn 100
In square planar complex of ion unpaired electrons are present in
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Coordination Compounds
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
(Atomic No. = Ti : 22, Cr : 24 and Mo : 42)
MEDIUM
HARD
EASY
MEDIUM
MEDIUM
MEDIUM
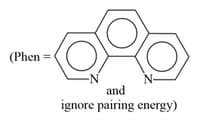
MEDIUM
MEDIUM
EASY

