MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
In the conductometric titration of vs , the titration curve obtained will be of the type:
(a)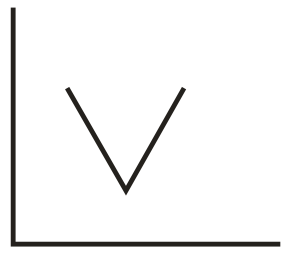

(b)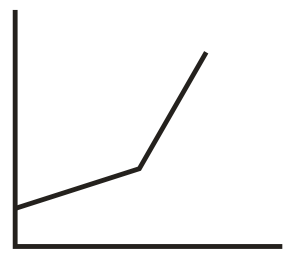

(c)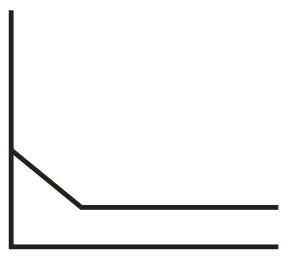

(d)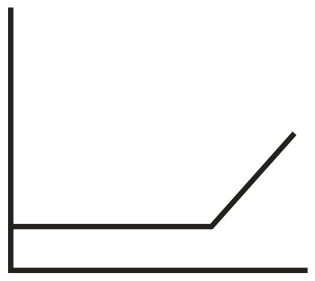

11.11% studentsanswered this correctly

Important Questions on Electrochemistry
MEDIUM
JEE Main/Advance
IMPORTANT
The standard potentials (in volt) corresponding to the reactions and are and respectively. The value (in volt) of the standard potential corresponding to the reaction is:
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
In an alkaline energy cell the overall cell reaction is as follows:
Which of the following reactions is taking place at the cathode?
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
