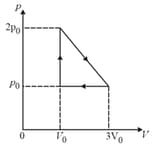
In the cyclic process shown in the figure, consider the following statements.
I. Area Work done on the gas
II. Area Net heat absorbed
III. Change in the internal energy in cycle
Which of these are correct?


Important Questions on The First Law of Thermodynamics
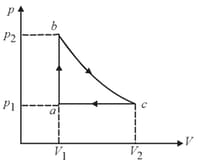
Carbon monoxide is carried around a closed cycle in which is an isothermal process as shown in the figure. The gas absorbs of heat as its temperature increases from to in going from to . The quantity of heat rejected by the gas during the process is approximately
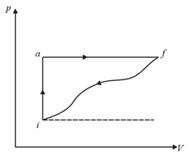
When a system is taken from state to a state along path , and . If for the curved return path in this path is
The diagram of a system undergoing thermodynamic transformation is as shown in figure. The work done by the system in going from is and heat is given to the system. The change in internal energy between and is
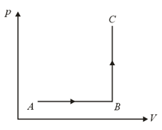
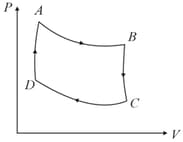
diagram of a cyclic process is as shown in figure. Choose the correct statement.
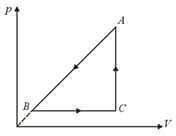
A sample of an ideal gas is taken through a cycle as shown in figure. It absorbs of energy during the process , no heat during , rejects during . of work is done on the gas during . Internal energy of gas at is the internal energy at would be
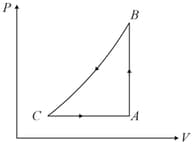
An ideal mono atomic gas is taken around the cycle as shown in diagram. The work done during the cycle is given by