HARD
JEE Main/Advance
IMPORTANT
Earn 100
In the following conversion, , the nitrogen atom changes its state of hybridisation from
(a) to .
(b) to .
(c) to .
(d) to .
60.71% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main/Advance
IMPORTANT
Define bond order.
EASY
JEE Main/Advance
IMPORTANT
Explain the significance of formal charge.
MEDIUM
JEE Main/Advance
IMPORTANT
Which one of the following has highest dipole moment?
HARD
JEE Main/Advance
IMPORTANT
The correct order of dipole moment for the following molecules is:
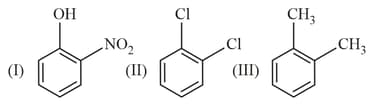
HARD
JEE Main/Advance
IMPORTANT
The correct order of dipole moment for the following molecules is:

MEDIUM
JEE Main/Advance
IMPORTANT
On the basis of intermolecular forces predict the correct order of decreasing boiling points of the compounds -
HARD
JEE Main/Advance
IMPORTANT
The reaction is endothermic. What can be concluded about the average per bond in and ?
MEDIUM
JEE Main/Advance
IMPORTANT
The electron-pair geometry of the central oxygen atom of ozone is:
