HARD
JEE Main
IMPORTANT
Earn 100
In the following reaction
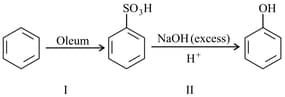
The yield for reaction is and that of reaction II is . The overall yield of the complete reaction is

77.78% studentsanswered this correctly

Important Questions on Some Basic Concepts of Chemistry
MEDIUM
JEE Main
IMPORTANT
The normality of in the solution obtained on mixing of with of is____.
MEDIUM
JEE Main
IMPORTANT
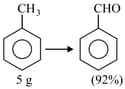
In the above reaction, of toluene is converted into benzaldehyde with yield. The amount of benzaldehyde produced is____
MEDIUM
JEE Main
IMPORTANT
In the given reaction,
if one mole of each of and with of gives compound . (Given : Atomic masses of and are 10,20 and , respectively). The yield of is____.
HARD
JEE Main
IMPORTANT
On complete combustion of of an organic compound containing and of and of was produced. The composition of oxygen in the compound is____. (Nearest Integer)
MEDIUM
JEE Main
IMPORTANT
of is reacted with of solution, the molarity of the resulting product in the solution is____millimolar.
MEDIUM
JEE Main
IMPORTANT
Consider the above reaction, the limiting reagent of the reaction and number of moles of formed respectively are
HARD
JEE Main
IMPORTANT
In bromination of Propyne, with Bromine -tetrabromopropane is obtained in yield. The amount of tetrabromopropane obtained from of Bromine in this reaction is_____ . (Molar Mass : Bromine )
MEDIUM
JEE Main
IMPORTANT
Consider the reaction
The amount of required to produce of is
(Given : Atomic masses of and are and , respectively.)
