EASY
Earn 100
In the formation of a chlorine molecule, orbital of one chlorine molecule overlaps with the orbital of another chlorine atom.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Chemical Bonding
MEDIUM
MEDIUM
EASY
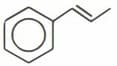
What is the total number of sigma and pi bonds in the following molecule?
MEDIUM
MEDIUM
MEDIUM
What is the total number of sigma and pi bonds in the following molecule?
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
Considering -axis as the internuclear axis, which out of the following will not form a sigma bond and why?
(a) and (b) (c) (d) .
MEDIUM
MEDIUM
HARD