HARD
JEE Main/Advance
IMPORTANT
Earn 100
In the given diagram, if temperature at is then maximum temperature during the process is_____
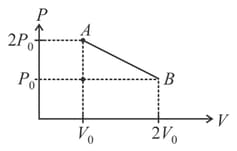

50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
JEE Main/Advance
IMPORTANT
A given mass of ideal gas changes its state from to along three different paths and If and be the heat supplied to the gas along respective paths then
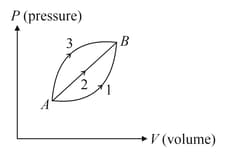
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Figure shows diagram for moles of an ideal gas. The volume of the gas is nearly
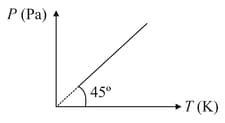
EASY
JEE Main/Advance
IMPORTANT
The curve for mole of an ideal monoatomic gas during process is shown in figure. The change in internal energy of the gas is
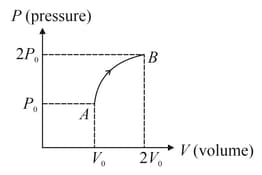
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
