EASY
NEET
IMPORTANT
Earn 100
In which of the following molecules is the nitrogen atom hybridised?
(a)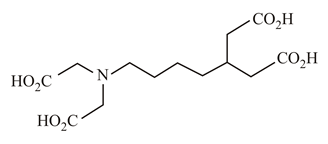

(b)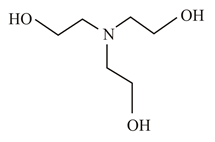

(c)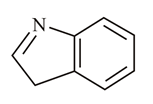

(d)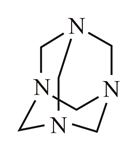

50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
What happens to bond angle when reacts with ?
EASY
NEET
IMPORTANT
Which oxide of is paramagnetic?
EASY
NEET
IMPORTANT
In beryllium chloride the hybridization possible in beryllium is:
HARD
NEET
IMPORTANT
Column A describe nature of bonding and Column B the solid having that type of bonding:
| A Nature of bonding) | B (solid) | ||
| I | Van der Waals | P | |
| II | lonic | Q | |
| III | Metallic | R | , diamond |
| IV | Covalent | S | |
Correct matching of A and B is in alternate:
EASY
NEET
IMPORTANT
Hybridization of orbitals of carbon in is necessary to explain which of the following:
EASY
NEET
IMPORTANT
In which of the following, '' atom is hybridized:
MEDIUM
NEET
IMPORTANT
The hybridization of carbon atoms in single bond of is:
EASY
NEET
IMPORTANT
, the hybridization state of Carbon is:
