Liquefied natural gas(LNG) is mainly methane. A tank is constructed to store LNG at and pressure, under this condition density of LNG is . The volume of a storage tank capable of holding the same mass of LNG as a gas, at and pressure is, . What is the value of ?
Important Questions on States of Matter: Gaseous and Liquid States
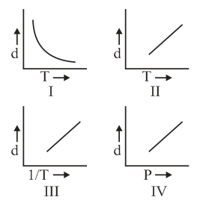
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
One moles each of four ideal gases are kept as follows.
I. of gas at pressure
II. of gas at pressure
III. of gas at pressure
IV. of gas at pressure
Which of the above gases is kept at highest temperature.
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
A container of volume can with stand a maximum pressure of atm at before exploding. The maximum amount of nitrogen (in ) that can be safely put in this container at this temperature is closest to