List the important products of the chlor-alkali process. Write one important use of each.

Important Questions on Acids, Bases and Salts
Which of the options in the given table are correct?
| Option | Natural Source | Acid Present |
| (i) | Orange | Oxalic acid |
| (ii) | Sour milk | Lactic milk |
| (iii) | Ant sting | Methanoic acid |
| (iv) | Tamarind | Acetic acid |
Study the following table and choose the correct option:
| Salt | Parent Acid | Parent Base | Nature of Salt | |
| a | Sodium Chloride | Basic | ||
| b | Sodium Carbonate | Neutral | ||
| c | Sodium Sulphate | Acidic | ||
| d | Sodium Acetate | Basic |
Consider the pH value of the following acidic samples:
| S.No. | Sample | pH Value |
| 1. | Lemon juice | |
| 2. | Gastric juice | |
| 3. | Vinegar | |
| 4. | Dil.Acetic acid |
The decreasing order of their ion concentration is:
Study the experimental set-up shown in given figure and chose the correct option from the following:
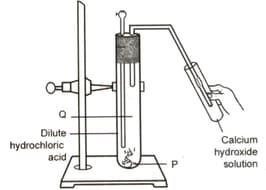
Which of the following salts do not have the water of crystallisation?
(i) Bleaching powder
(ii) Plaster of Paris
(iii) Washing soda
(iv) Baking soda
of tap water was taken in a beaker. Hydrochloric acid was added drop by drop to water. The temperature and pH of the solution was noted. The following graph was obtained. Choose the correct statements related to this activity.
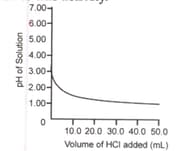
(i) The process of dissolving an acid in water is highly endothermic
(ii) The pH of the solution increases rapidly on addition of acid.
(iii) The pH of the solution decreases rapidly on addition of acid.
(iv) The pH of tap water was around .
