Look at the list of metals. Carbon can be placed between zinc and aluminium. Will carbon aluminium oxide will react?
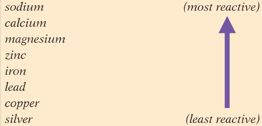
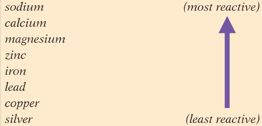

Important Questions on The Behaviour of Metals
Look at the list of metals. Carbon can be placed between zinc and aluminium. Will carbon copper oxide will react?
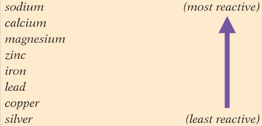
Look at the list of metals. Carbon can be placed between zinc and aluminium. Will magnesium carbon dioxide will react?
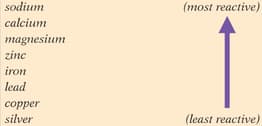
Write a word equation for the following two reactions, and name the substance that is reduced.
- The reaction of carbon copper oxide.
- The reaction of magnesium carbon dioxide
When magnesium powder is added to copper sulphate solution, a displacement reaction occurs and solid appear forms. Write a word equation for the reaction.
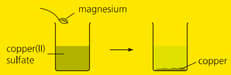
When magnesium powder is added to copper sulphate solution, a displacement reaction occurs and solid appear forms. Why does the displacement reaction occur.
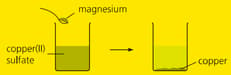
When magnesium powder is added to copper sulphate solution, a displacement reaction occurs and solid appear forms. Write a half-equation to show what happens to the magnesium atoms.

When magnesium powder is added to copper sulphate solution, a displacement reaction occurs and solid appear forms. Write a half-equation to show what happens to the magnesium atoms. Which type of reaction is this?

When magnesium powder is added to copper sulphate solution, a displacement reaction occurs and solid appear forms. Write a half-equation to show what happens to the copper atoms.

