EASY
AS and A Level
IMPORTANT
Earn 100
Look at the mass spectrum of ethanol, . A structural isomer of ethanol is methoxy methane, an ether with the formula .
Predict and give the name of the fragment that would appear on the mass spectrum of methoxy methane but does not appear on methanol's mass spectrum.
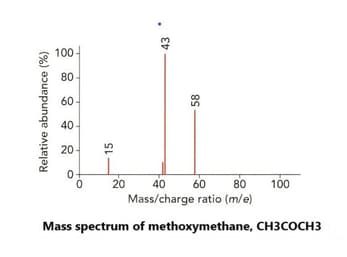
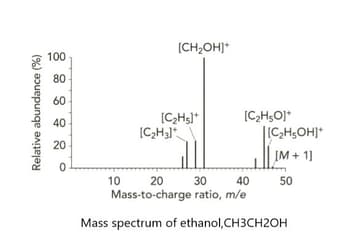



Important Questions on Atoms, Molecules and Stoichiometry
EASY
AS and A Level
IMPORTANT
Use the value of sulphur is to calculate the amount of substance in moles in of sulphur atoms.
EASY
AS and A Level
IMPORTANT
Use the value of sulphur is to calculate the amount of substance in moles in of sulphur molecules .
EASY
AS and A Level
IMPORTANT
Use these values to calculate the amount of substance in moles in of anhydrous iron(III) nitrate, .
EASY
AS and A Level
IMPORTANT
Use the value of the Avogadro constant to calculate the total number of atoms in of chlorine atoms. ( value: ).
EASY
AS and A Level
IMPORTANT
Use these values: .
Calculate the mass of moles of carbon dioxide, .
EASY
AS and A Level
IMPORTANT
Use these values: .
Calculate the mass of moles of sodium carbonate, .
EASY
AS and A Level
IMPORTANT
Use these values: .
Calculate the mass of moles of iron(II) hydroxide, .
EASY
AS and A Level
IMPORTANT
Tin(IV) oxide is reduced to tin by carbon. Carbon monoxide is also formed.
Calculate the mass of carbon that exactly reacts with of tin(IV) oxide. Give your answer to significant figures.
( values: ).
