EASY
Earn 100
Lower ethers are appreciably soluble in water.
(a)True
(b)False
94.12% studentsanswered this correctly
Important Questions on Alcohols, Phenols, and Ethers
MEDIUM
Why ether is insoluble in water?
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
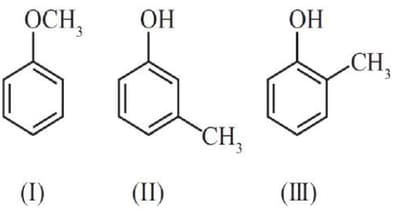
HARD
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
HARD
MEDIUM
EASY
EASY
HARD
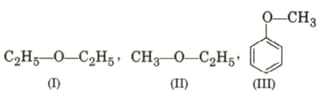
EASY
MEDIUM

