EASY
Earn 100
Match the column
Column
(Arrangement of the atoms/ions)
Column
(Planes in fcc lattice)

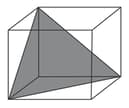
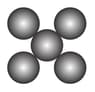
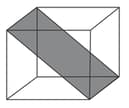

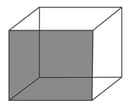

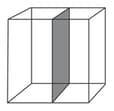
Column
(Arrangement of the atoms/ions)
Column
(Planes in fcc lattice)








(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Solid State
EASY
EASY
MEDIUM
EASY
EASY
EASY

EASY
EASY
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
HARD

