EASY
TS EAMCET
IMPORTANT
Earn 100
Molality is defined as
(a)Number of moles of solute in of solvent
(b)Number of moles of solute in of solvent
(c)Number of moles of solute in moles of solvent
(d)Number of moles of solute in one litre of solvent
80.95% studentsanswered this correctly

Important Questions on Solutions
EASY
TS EAMCET
IMPORTANT
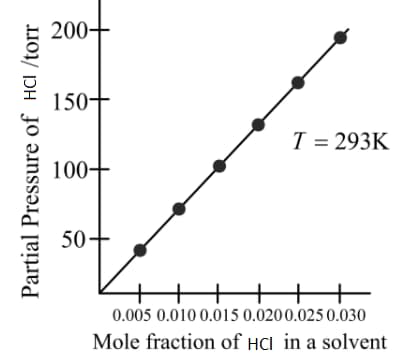
From the graph, the value of Henry's constant for the solubility of gas in cyclohexane is
MEDIUM
TS EAMCET
IMPORTANT
A solution contains moles of and moles of , and has a normal boiling point of . The vapor pressure of pure is . What is the vapor pressure of pure at this temperature?
MEDIUM
TS EAMCET
IMPORTANT
Which of the following mixtures shows negative deviation from Raoult's law?
EASY
TS EAMCET
IMPORTANT
Which of the following pair shows a positive deviation from Raoult's law?
MEDIUM
TS EAMCET
IMPORTANT
The vapour pressure of a solution of glucose is at . What is the mole fraction of water in this solution?
MEDIUM
TS EAMCET
IMPORTANT
of glucose, is dissolved in of water in a saucepan. At what temperature the solution will boil at bar?
for water is
EASY
TS EAMCET
IMPORTANT
Which of the following aqueous solution has highest boiling point? (Assume identical conditions)
EASY
TS EAMCET
IMPORTANT
Which one of the following graphs correctly represents change in freezing point as a function of solute concentration?
