HARD
Earn 100
Molecular speed distributions for a gas at two different temperatures are shown below. Which of the graphs correctly describes the distributions at the two temperatures, where
(Note: small vertical lines indicate average speed.)
(a)
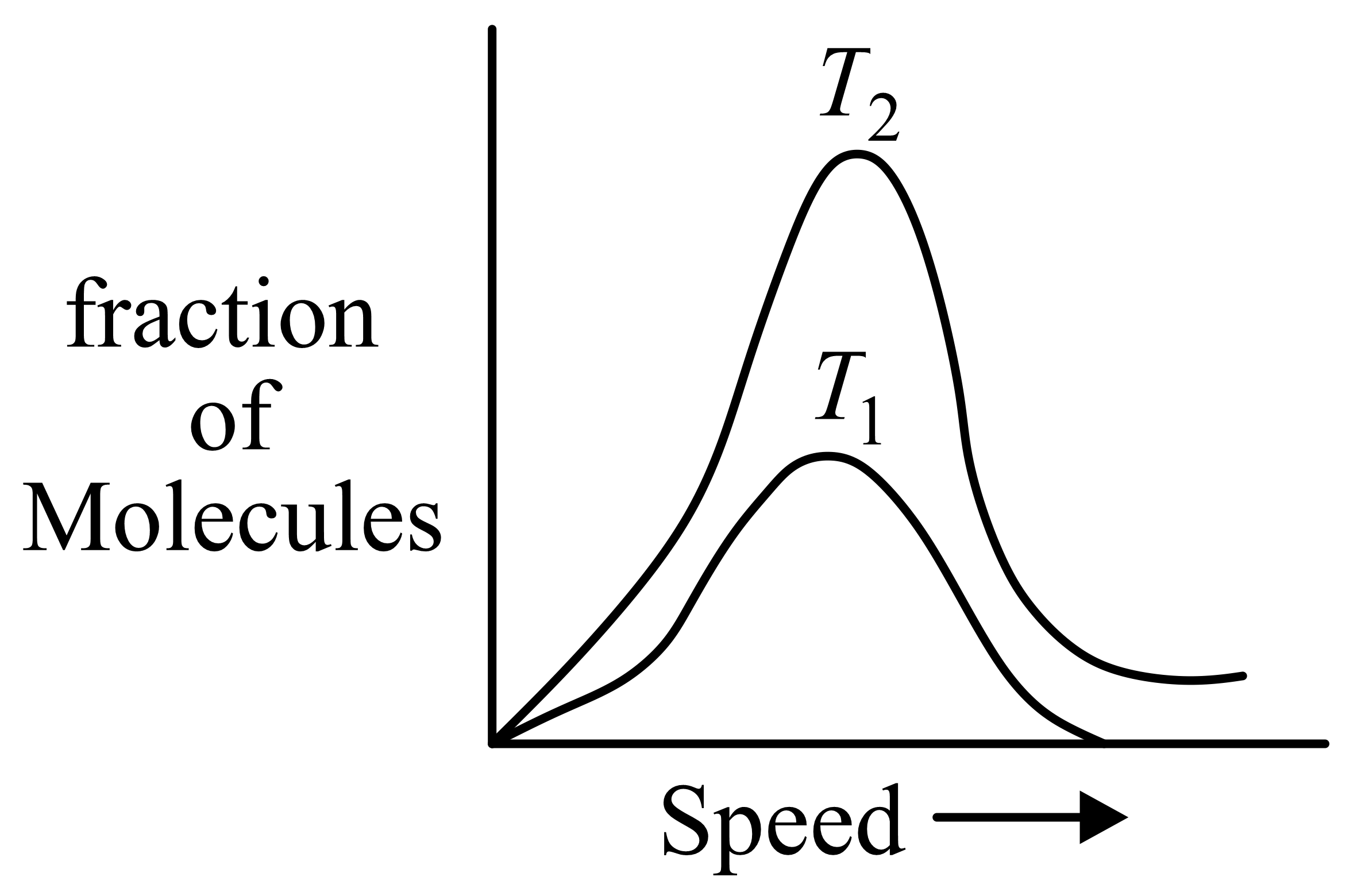
(b)
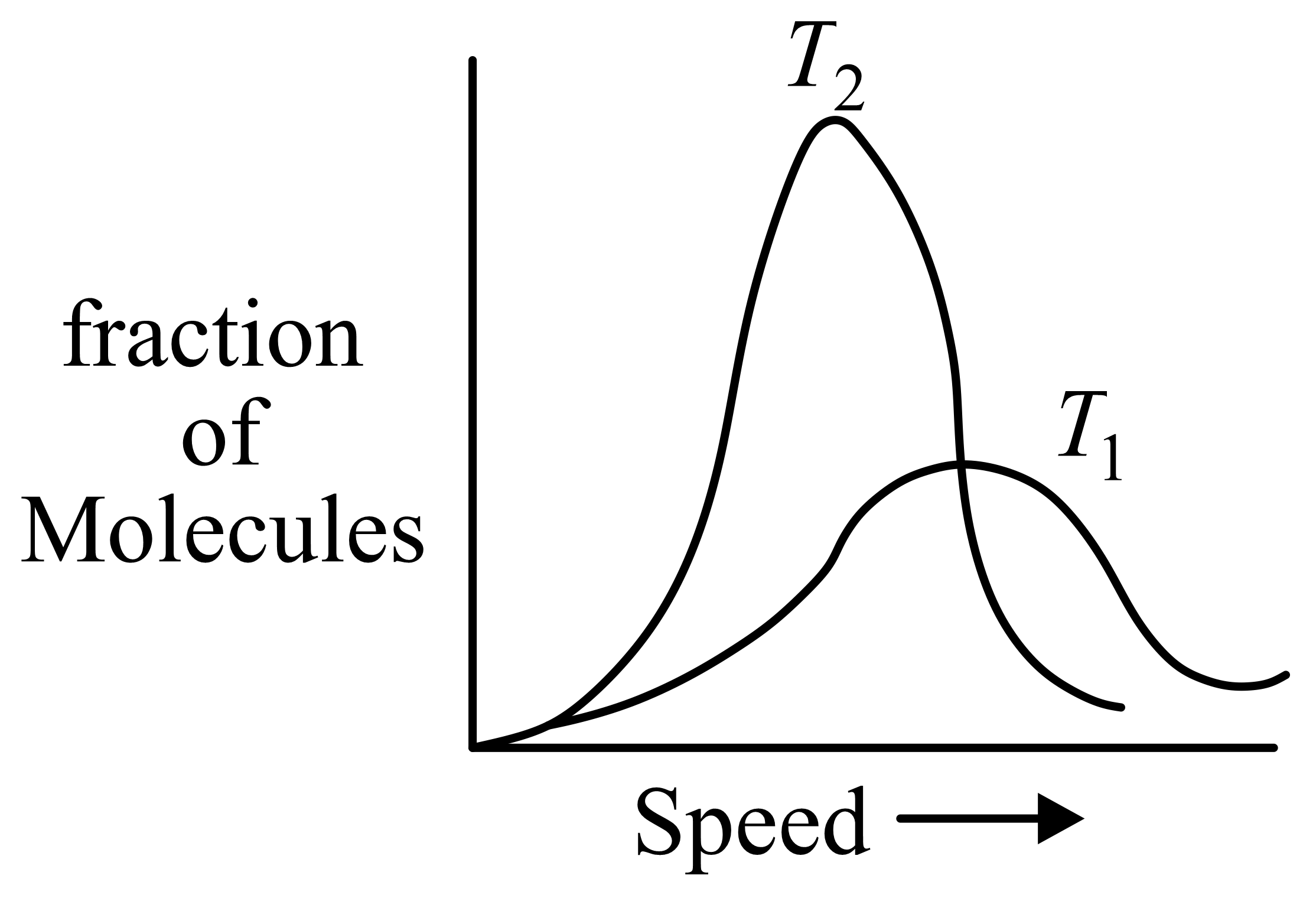
(c)
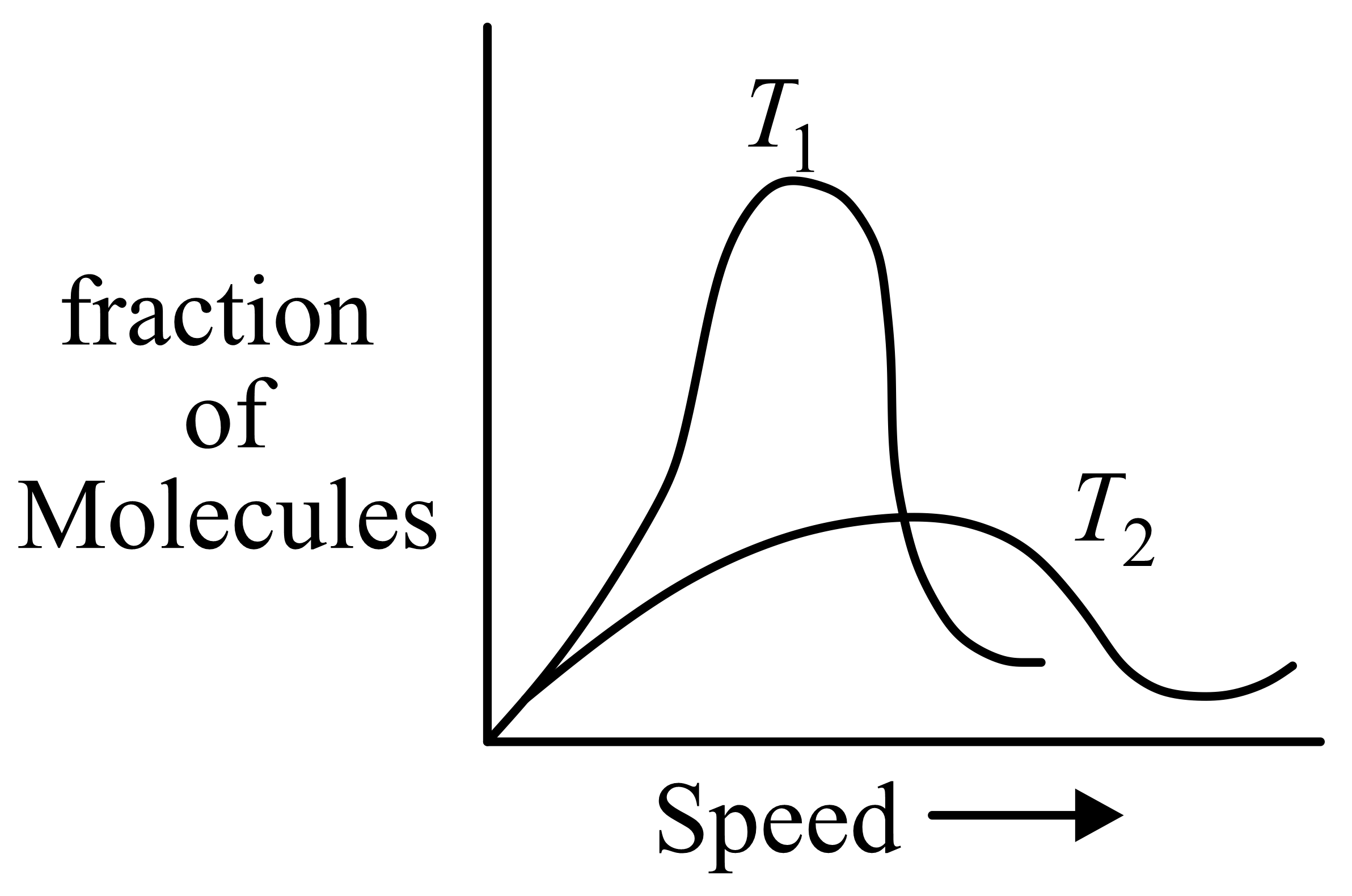
(d)
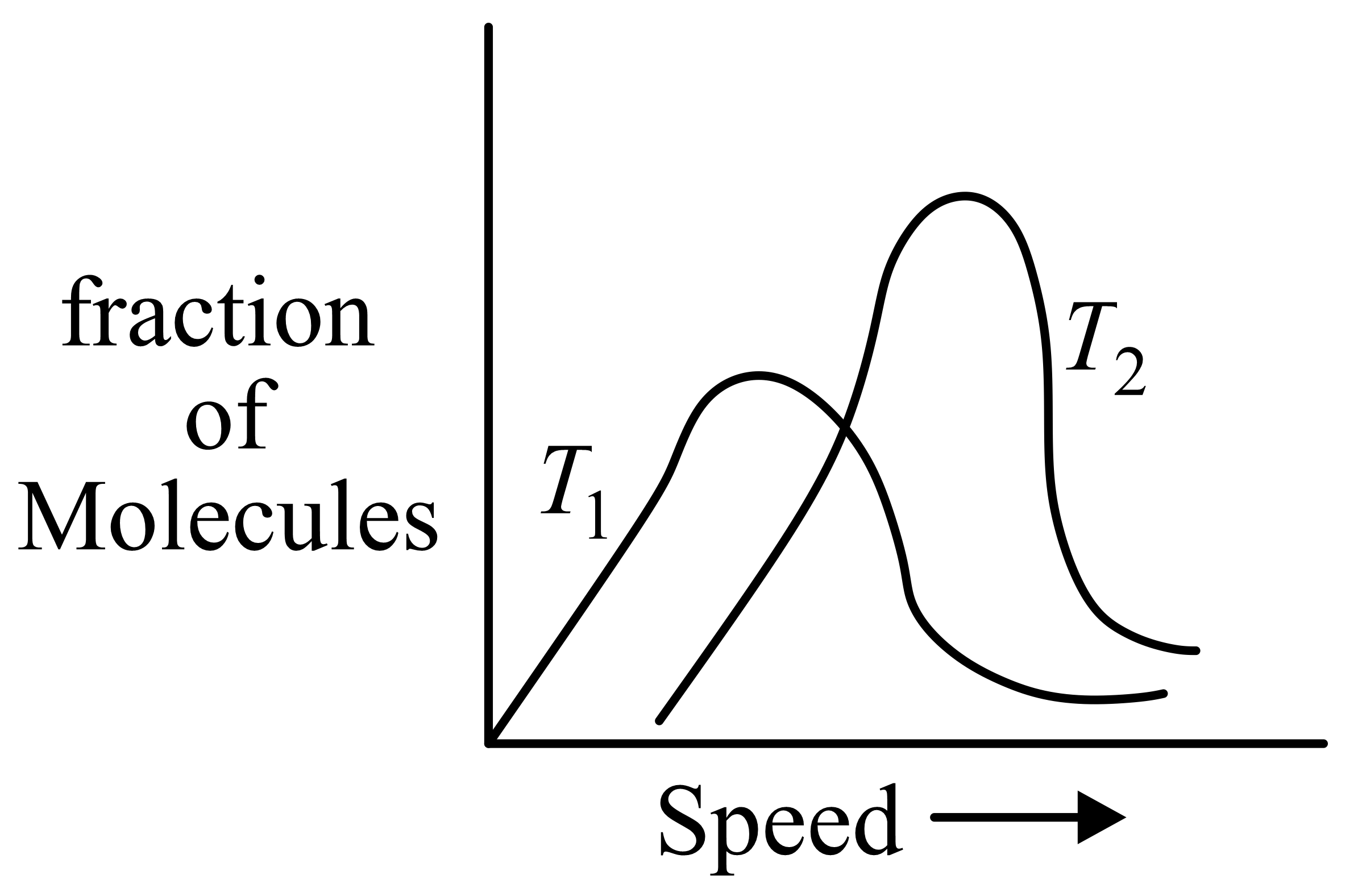
37.5% studentsanswered this correctly
Important Questions on Kinetic Theory of Gases
MEDIUM
HARD
Consider an ideal gas confined in an isolated closed chamber. As the gas undergoes an adiabatic expansion, the average time of collision between molecules increases as , where is the volume of the gas. The value of is:
EASY
EASY
HARD
MEDIUM
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
Boltzmann Constant
Avogadro number
Radius of Earth:
Gravitational acceleration on Earth
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
EASY

