EASY
NEET
IMPORTANT
Earn 100
is isoelectronic with and which is the structure of ?
(a)
(b)
(c)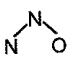

(d)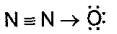
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
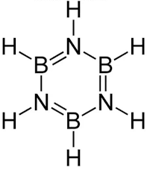
What are the formal charges on and , respectively in the given structure?
EASY
NEET
IMPORTANT
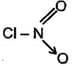
What is the formal charge on in;
EASY
NEET
IMPORTANT
For hydrazoic acid, which of the following resonating structure will be least stable?
EASY
NEET
IMPORTANT
Which of the following overlaps is incorrect [assuming axis to be the internuclear axis]?
(a)
(b)
(c)
(d)
EASY
NEET
IMPORTANT
Which of the following statements is correct about the bond?
EASY
NEET
IMPORTANT
overlapping will be observed in the molecule of:
MEDIUM
NEET
IMPORTANT
Identify the correct match.
| () | (a) | Central atom has hybridisation and bent geometry. | |
| () | (b) | Central atom has hybridisation and octahedral. | |
| () | anion | (c) | Central atom has hybridisation and linear geometry. |
| () | cation | (d) | Central atom has hybridisation and linear geometry. |
EASY
NEET
IMPORTANT
In which of the following molecules/species are all following characteristics are found:
(a) Tetrahedral hybridisation
(b) Hybridisation can be considered to have taken place with the help of empty orbital(s).
(c) All bond lengths are identical, i.e., all bond lengths are identical.
