HARD
11th CBSE
IMPORTANT
Earn 100
is dissociated at a total pressure and dissociated at a total pressure . Then ratio is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Equilibrium in Physical and Chemical Processes
HARD
11th CBSE
IMPORTANT
At the equilibrium of the reaction, , the observed molecular weight of is at . The percentage of dissociation of at is
MEDIUM
11th CBSE
IMPORTANT
The values of and for the reactions , and are in the ratio of . If the degree of dissociation of and be equal, then total pressure at equilibrium and are in the ratio
MEDIUM
11th CBSE
IMPORTANT
of and of are mixed together and allowed to come into equilibrium according to the following reaction
When equilibrium is reached, there is of . The equilibrium extent of the reaction is
MEDIUM
11th CBSE
IMPORTANT
Which of the following lines correctly show the temperature dependence of equilibrium constant , for an exothermic reaction?
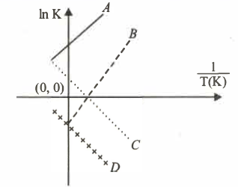
MEDIUM
11th CBSE
IMPORTANT
Which of the following are reversible reactions?
MEDIUM
11th CBSE
IMPORTANT
Which of the following statements are wrong?
MEDIUM
11th CBSE
IMPORTANT
The equilibrium is attained at in a closed container and inert gas helium is introduced. Which of the following statements are correct?
HARD
11th CBSE
IMPORTANT
For the reaction , the forward reaction at constant temperature is favoured by
