EASY
Earn 100
is dissociated to 33% and 40% at total pressure and atm respectively. Then the ratio is
(a)7/4
(b)7/3
(c)8/3
(d)8/5
50% studentsanswered this correctly
Important Questions on Equilibrium
EASY
.....(1)
.....(2)
The relation between and is:
MEDIUM
When and are compared at It is found that
HARD
(At )
EASY
MEDIUM
EASY
HARD
MEDIUM
EASY
The rate of this reaction is increased by
EASY
For the reaction:
is
HARD
MEDIUM
The value of for the reaction
The value of for the following reaction is :
MEDIUM
The equilibrium constant () of the reaction:
, will be:
MEDIUM
MEDIUM
.... (i)
.... (ii)
are related as
EASY
(assuming ideality)
MEDIUM
MEDIUM
moles each of hydrogen and iodine is heated in a sealed ten litre vessel.At equilibrium, moles of were found. The equilibrium constant for is ........
HARD
The reaction
is in equilibrium in a closed vessel at The partial pressure (in atm) of in the reaction vessel is closest to:
[Given: The change in Gibbs energies of formation at and bar for
Gas constant ]
EASY
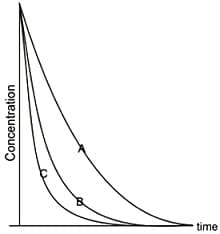
These profiles imply that the decay constants and follow the order

