MEDIUM
Earn 100
Name the different types of electrodes?
Important Questions on Electrochemistry
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
Represent a cell consisting of | half cell and | half cell and write the cell reaction.
EASY
EASY
At , the of the galvanic cell mentioned below is
EASY
HARD
The reaction occurs in which of the given galvanic cell?
EASY
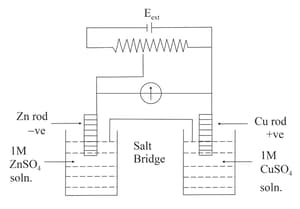
Identify the incorrect statement from the options below for the above cell:
EASY
MEDIUM
Some materials are given below:
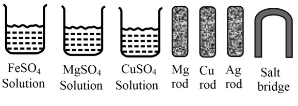
From the given materials, choose the appropriate ones to construct a galvanic cell and draw the diagram of the cell.
(Order of reactivity: )
EASY
HARD
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
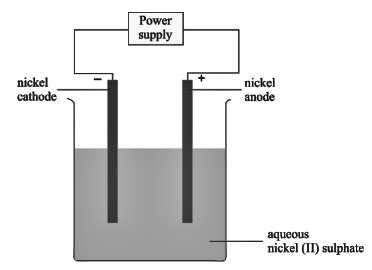
- Which equation for the reaction at the anode is correct?
EASY
MEDIUM
Depict the galvanic cell in which the reaction takes place. Further show, which of the electrode is negatively charged.
.
MEDIUM
MEDIUM

