Name the product obtained when ethanol is oxidized by either chromic anhydride or alkaline potassium permanganate.
Important Questions on Carbon and its Compounds
Describe the industrial production of ethanol from molasses under the following head:
Labelled diagram
Nominate following compounds on the basis of organic compound nomenclature system.
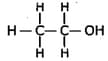
Answer the following:
Give a chemical equation for the reaction of Ethanol with excess hot conc. sulphuric acid.
Sugar is converted to alcohol.
(i) In the above reaction what kind of process takes place?
(ii) Which micro-organism is involved?
Study the equation and answer the following:
What is the role of in the above reaction?
"Manufacture of ethyl alcohol from molasses is a good example for fermentation." Give reasons.
Read each description given below and say whether it fits for ethanol or ethanoic acid.
(i) It is a clear liquid with a burning taste.
(ii) It is used to preserve biological specimens in laboratories.
Study the equation and answer the following:
What is the role of in the above reaction?
Name the following:
The residue is left behind when the wood is burnt in a limited supply of air.
Identify X, Y and Z.
Describe the industrial production of ethanol from molasses under the following head:
Chemical equation of the process
of ethanol is taken in a test tube and warmed gently in a water bath. A solution of alkaline potassium permanganate is added first, drop by drop to this solution, then in excess.
- How is solution of prepared?
- State the role of alkaline potassium permanganate in this reaction. What happens on adding it in excess?
- Write a chemical equation of this reaction.

