EASY
Earn 100
Neutral ferric chloride is added to the aqueous solution of acetate ion. The blood-red colour obtained is due to the formation of:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Theoretical Principles of Experimental Chemistry
MEDIUM
A white sodium salt dissolves readily in water to give a solution which is neutral to litmus. When silver nitrate solution is added to the before mentioned solution, a white precipitate is obtained which does not dissolve in dilute nitric acid. The anion is:
EASY
In the detection of II group acid radical, the salt containing chloride is treated with concentrated sulphuric acid, the colourless gas is liberated. The name of the gas is
HARD
A white precipitate was formed when was added to water extract of an inorganic salt. Further, a gas with characteristic odour was released when the formed white precipitate was dissolved in dilute HCI. The anion present in the inorganic salt is:
EASY
When treated with conc. yields gas which further reacts with to generate a white solid reacts with dil. to produce the same gas the solid is
EASY
On heating, lead (ll) nitrate gives a brown gas (A). The gas (A) on cooling changes to a colourless solid/liquid (B). (B) on heating with NO changes to ablue solid (C). The oxidation number of nitrogen in solid (C) is:
MEDIUM
Which combines with to form brown complex?
MEDIUM
Match List I with List II.
| List-I (Anion) |
List-II (gas evolved on reaction with dil. ) |
||
| (A) | (I) |
Colourless gas which turns lead acetate paper black. |
|
| (B) | (II) |
Colourless gas which turns acidified potassium dichromate solution green. |
|
| (C) | (III) | Brown fumes which turns acidified KI solution containing starch blue. | |
| (D) | (IV) | Colourless gas evolved with brisk effervescence, which turns lime water milky. |
Choose the correct answer from the options given below
HARD
When a mixture of and concentrated is heated in a dry test tube, a red vapour is evolved. This vapour turns an aqueous solution of yellow due to the formation of and respectively, are:
EASY
Reaction of an inorganic sulphite with dilute generated compound . Reaction of with gives . Further, the reaction of with and water affords compound and , respectively, are :
MEDIUM
Sodium nitroprusside, when added to an alkaline solution of sulphide ions, produce a
MEDIUM
The sodium salt of an organic acid produces effervescence with concentrated . reacts with the acidified aqueous solution to give a white precipitate which decolourises acidic solution of . is
MEDIUM
and both when dissolved in water containing ions the pair of species formed is:
HARD
The reagent(s) that can selectively precipitate from a mixture of and in aqueous solution is (are)
MEDIUM
Some reactions are given for salt(X).
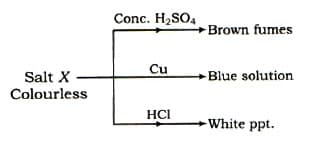
Which of the following salt can be satisfied all the conditions?
MEDIUM
Nitrite interferes in the 'ring-test' of nitrates . Among the following reagent(s) that can be used for the removal of nitrite is/are
I.
II. (Thiourea)
III. (Sulphamic acid)
Correct choice is
HARD
Select the incorrect statement.
MEDIUM
When NaCl, is heated with solid K2Cr2O7 & Conc. H2SO4 the vapours obtained is
MEDIUM
Corrosive sublimate can be used to distinguish between:
HARD
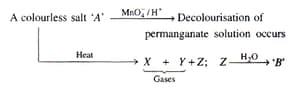
Gas
Gas X burns with blue flame. Mark the correct choice.
EASY
Brown ring is made for

