Note: Answer the question on the basis of information given below:
“Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged carbon atom involvement of neighboring groups in hyperconjugation and resonance.”
The structure of triphenylmethyl cation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of high stability of this cation.
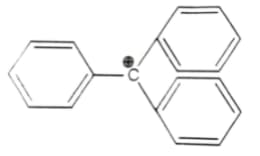
“Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged carbon atom involvement of neighboring groups in hyperconjugation and resonance.”


Important Questions on Organic Chemistry – Some Basic Principles and Techniques
Write structures of various carbocations that can be obtained from -methylbutane. Arrange these carbocation’s in order of increasing stability.
Name the compound whose line formula is given below
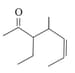
Name the compound whose line formula is given below:
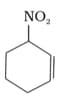
Write structural formulae for compound named as-
-Bromoheptane
Write structural formulae for compound named as-
-Bromoheptanoic acid
Draw the resonance structures of the following compound;
Draw the resonance structures of the following compound;
