Number of incorrect statements are:
1. Glucose is aldohexose
2. Glucose have many isomeric forms in aqueous medium
3. Glucose is soluble in water due to the presence of an aldehyde functional group
4. Glucose is a reducing sugar
Important Questions on Biomolecules
Consider the following reactions :
(i) Glucose acetyl derivative
(ii) Glucose acetyl derivative
(iii) Glucose acetyl derivative '' , '' and '' in these reactions are respectively.
Identify the product in following reaction
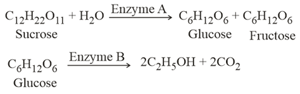
In the above reactions, the enzyme and enzyme B respectively are :-
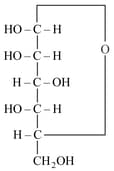
For the below given cyclic hemiacetal , the correct pyranose structure is
What is the relationship between the given structures (Look at the arrows)
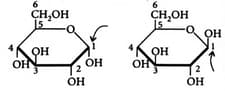
Number of stereo centers present in linear and cyclic structures of glucose are respectively:

