Observe the given carefully.
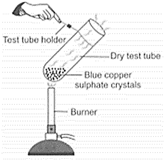
Which of the following observations are correct?
I. A white coloured residue is left behind in the test tube.
II. Water droplets are observed on the upper cooler part of the test tube.
III. On adding water to the residue, the colour changes to green.


Important Questions on Acids, Bases and Salts
Read the given passage and fill in the blanks by selecting an appropriate option.
Bleaching powder is a I powder. When exposed to air, it reacts with II of the air to liberate gas. It is III in cold water and the milkiness of the solution is due to the presence of unreached IV. It reacts with and liberating V gas.
| (i) | (ii) | (iii) | (iv) | ||
| (A) | Blue crystalline | Moisture | Soluble | ||
| (B) | Yellowish white | Soluble | Lime | ||
| (C) | White | Insoluble | lime | ||
| (D) | Yellow | Moisture | Soluble |
Observer the given experimental set-up carefully.
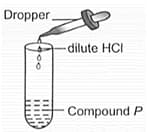
Which of the following could be the possible observation and inference drawn?
Few statements regarding the properties of bases are given below. Mark the correct statements?
(i) Sodium hydroxide and potassium hydroxide are soluble in water.
(ii) Calcium hydroxide and magnesium hydroxide are partially soluble in water.
(iii) All metallic hydroxides react with acids to form their respective metallic salts.
(iv) Metallic oxides are acidic oxides hence they react with acids to form salts.Read the given statements and mark the correct option.
Statement 1: A solution of has hydrogen ion concentration times than that of solution of .
Statement 2:A metal carbonate on reacting with acid gives a gas which when passed through a solution gives the carbonate back. Solution reacts with gas to form a compound used for decolourisation. Identify
| W | X | Y | Z | P | |
| (A) | |||||
| (B) | |||||
| (C) | |||||
| (D) |
