On the basis of electrochemical theory of corrosion, in aqueous solution reaction occuring at the cathode is.
Important Questions on Electrochemistry
Some materials are given below:
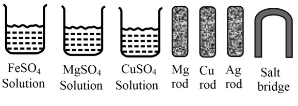
From the given materials, choose the appropriate ones to construct a galvanic cell and draw the diagram of the cell.
(Order of reactivity: )
Some materials are given below:
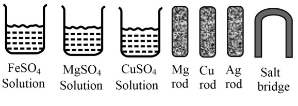
Write the chemical equation fo the reaction taking place at the cathode.
Some materials are given below:
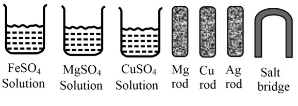
Which is the anode of this cell?
,
Depict the galvanic cell in which the reaction takes place. Further show, which of the electrode is negatively charged.
.
At , the of the galvanic cell mentioned below is
The reaction occurs in which of the given galvanic cell?
For the cell , when the concentration of is times the concentration of , the expression for is
Faraday's constant, universal gas constant, temperature,
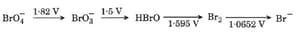
Then, what is the species undergoing disproportionation?
The following reaction takes place at in an electrochemical cell involving two metals and ,
with and in the respective half-cells, the cell EMF is . The equilibrium constant of the reaction is closest to;
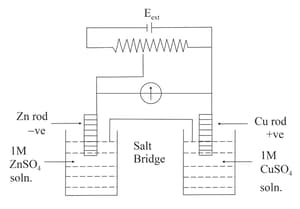
Identify the incorrect statement from the options below for the above cell:

