EASY
Earn 100
On the basis of the figure given below, answer the following question:
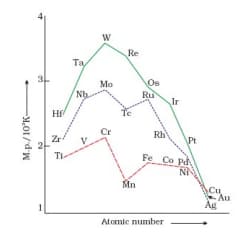
In the third transition series, identify and name the metal with the highest melting point.

Important Questions on The d- and f-Block Elements
EASY
| Catalyst | Process | ||
| A. | i. | Wacker process | |
| B. | ii. | Ziegler – Natta polymerisation | |
| C. | iii. | Contact process` | |
| D. | iv. | Deacon’s process |
HARD
EASY
Assertion: In general, transition metals have high melting points.
Reason: More number of electrons from '' and '' are involved in interatomic metallic bonding.
The correct option among the following is
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
HARD
(Atomic Number of )
EASY
Iron has higher enthalpy of atomization than that of copper.
EASY
MEDIUM
HARD
EASY
EASY
EASY
MEDIUM
or (High melting and boiling point)
EASY

