MEDIUM
Physics
IMPORTANT
Earn 100
One mole of a diatomic gas undergoes a process where and are constants. The translational kinetic energy of the gas when is given by
(a)
(b)
(c)
(d)
59.09% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
EASY
Physics
IMPORTANT
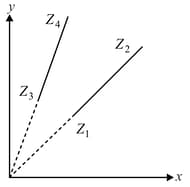
In the diagram as shown, find parameters representing and axes and also parameter , if and .
EASY
Physics
IMPORTANT
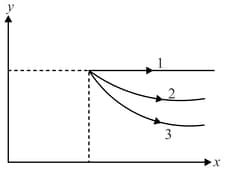
A diagram is shown below. Choose the corresponding diagram.
EASY
Physics
IMPORTANT
If the mean free path of atoms is doubled then the pressure of gas will become
EASY
Physics
IMPORTANT
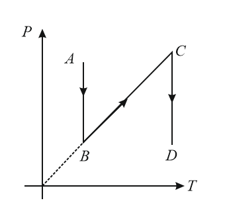
For process, is positive, for process , is zero and for process , is negative. Find the parameters indicating and axes.
HARD
Physics
IMPORTANT
At room temperature , the rms speed of the molecules of a certain diatomic gas is found to be . The gas is:
MEDIUM
Physics
IMPORTANT
A cubic vessel (with faces horizontal + vertical) contains an ideal gas at NTP. The vessel is heing carried by a rocket which is moving at a speed of in vertical direction. The pressure of the gas inside the vessel as observed by us on the ground
MEDIUM
Physics
IMPORTANT
mole of an ideal gas is contained in a cubical box of volume , at (figure). One face of the box is made up of a material which totally absorbs any gas molecule incident on it.
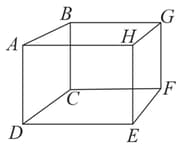
At any given time,
MEDIUM
Physics
IMPORTANT
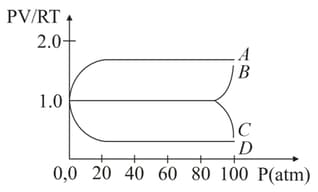
An experiment is carried on a fixed amount of gas at different temperatures and at high pressure such that it deviates from the ideal gas behaviour. The variation of with is shown in the diagram. The correct variation will correspond to