MEDIUM
WBJEE
IMPORTANT
Earn 100
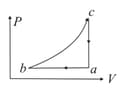
One mole of a monoatomic ideal gas undergoes a quasistatic process, which is depicted by a straight line joining points and in a diagram. What is the value of the heat capacity of the gas at the point
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
WBJEE
IMPORTANT
It rejects of heat during and absorbs of heat during . During , there is no transfer of heat and of work is done by the gas. What should be the area of the closed curve
HARD
WBJEE
IMPORTANT
The initial pressure and volume of a given mass of an ideal gas with , taken in a cylinder fitted with a piston, are and respectively. At this stage the gas has the same temperature as that of the surrounding medium which is It is adiabatically compressed to a volume equal to
Subsequently the gas is allowed to come to thermal equilibrium with the surroundings. What is the heat released to the surrounding?
EASY
WBJEE
IMPORTANT
MEDIUM
WBJEE
IMPORTANT
