Out of following, which molecules has same weight under identical volume at STP?

Important Points to Remember in Chapter -1 - Some Basic Concepts of Chemistry from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Matter and its Nature:
Based on their physical state and chemical characteristics, matter is classified as :
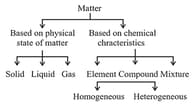
These three states of matter are interconvertible by changing the conditions of temperature and pressure, i.e., they change their state.
Based on the chemical composition, matter can be classified as:
(i) Element: Lavoisier gave the explanation for element. According to him, an element consists of only one type of atoms. In different elements, atoms are different. The atoms of one element are different from the atoms of the other element. Thus, every element has its own independent property, which is not the same in other elements, e.g., carbon, sodium, oxygen etc.
(ii) Compound: When two or more elements combine chemically with one another, a compound is formed. When the compounds are formed, the elements present in them show new type of properties by losing their own individual properties. For example, hydrogen and oxygen are gases. Water , formed by their combination, is a liquid. Here, hydrogen burns explosively in the air and oxygen is a supporter of combustion; but water is used as a fire extinguisher. Thus, hydrogen and oxygen change their properties in water.
(iii) Mixture: A mixture is a material consisting of two or more kinds of matter, each retaining its own characteristic property. A mixture can be separated by physical methods.
2. Measurement of Matter and the Uncertainty in Measurement:
(i) SI units (Basic units):
| Physical quantity | Symbol for quantity | Symbol of SI units | Name of the unit |
| Length | Metre | ||
| Mass | kilogram | ||
| Time | Second | ||
| Electric current | ampere | ||
| Thermodynamic temperature | Kelvin | ||
| Amount of substance | mole | ||
| Luminous intensity | candela |
(ii) Prefixes used in the SI system:
| Multiple | Prefix | Symbol |
| Femto | ||
| Pico | ||
| Nano | ||
| Micro | ||
| Milli | ||
| Centi | ||
| Deci | ||
| Deca | ||
| Hecta | ||
| Kilo | ||
| Mega | ||
| Giga | ||
| Tera | ||
| Peta |
3. Significant Figures:
(i) There are three rules on determining how many significant figures are in a number:
(a) Non-zero digits are always significant.
(b) Any zeros between two significant digits are significant.
(c) A final zero or trailing zeros in the decimal portion only are significant.
(ii) The following rules dictate the way the numbers are to be rounded to the number of figures indicated:
(a) When rounding, examine the figure following (i.e., to the right of) the figure that is to be the last. This figure you are examining is the first figure to be dropped.
(b) If it is less than , drop it and all the figures to the right of it.
(c) If it is more than , increase by the number to be rounded, that is, the preceding figure.
(d) If it is , round the number so that it will be even. Keep in mind that zero is even when rounding off.
(iii) Addition and Subtraction with significant numbers:
For addition and subtraction, look at the decimal portion (i.e., to the right of the decimal point) of the numbers ONLY.
(a) Count the number of significant figures in the decimal portion of each number in the problem. (The digits to the left of the decimal place are not used to determine the number of decimal places in the final answer.)
(b) Add or subtract in the normal fashion.
(c) Round the answer to the LEAST number of places in the decimal portion of any number in the problem.
(iv) Multiplication and Division with significant numbers:
The LEAST number of significant figures in any number of the problem determines the number of significant figures in the answer.
4. Laws of Chemical Combinations:
The following are the laws which govern the formation of chemical compounds:
(i) Law of Conservation of Mass:
"Matter can neither be created nor destroyed." This law was put forth by Antoine Lavoisier in 1789.
(ii) Law of Constant Proportion:
"A given compound always contains exactly the same proportion of elements by weight."
Joseph Proust observed that samples of cupric carbonate obtained naturally and prepared synthetically in the laboratory had the same percentage composition of elements.
| Cupric carbonate | of | of | of |
| Natural sample | |||
| Synthetic sample |
(iii) Law of Multiple Proportion:
This law was proposed by Dalton in 1803. If two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in the ratio of small whole number.
(iv) Gay Lussac’s Law of Gaseous Volumes:
This law was given by Gay Lussac in 1808. He observed that when gases combine or are produced in a chemical reaction, they do so in a simple ratio by volume, provided all gases are at the same temperature and pressure.
(v) Avogadro’s Law:
In , Avogadro proposed that equal volumes of all gases at the same temperature and pressure should contain equal number of molecules. Avogadro made a distinction between atoms and molecules which is quite understandable in the present times.
5. Dalton’s Atomic Theory:
In , Dalton published ‘A New System of Chemical Philosophy’, in which he proposed the following:
(i) Matter consists of indivisible atoms.
(ii) All atoms of a given element have identical properties, including identical mass. Atoms of different elements differ in mass.
(iii) Compounds are formed when atoms of different elements combine in a fixed ratio.
(iv) Chemical reactions involve reorganization of atoms. These are neither created nor destroyed in a chemical reaction.
Dalton’s theory could explain the laws of chemical combination.
6. Atomic and Molecular Mass:
(i) Atomic mass:
It is the average relative mass of its atoms as compared with an atom of carbon- isotope taken as .
The mass of atom atomic mass (in ).
The mass of mole atoms atomic mass (in ).
Example: The mass of one atom .
The mass of mole atoms .
(ii) Calculation of average atomic mass:
If an element exists in two isotopes having atomic masses ' and in the ratio , then:
Average atomic mass
(iii) Molecular mass:
Molecular mass of a substance is the average relative mass of its molecules as compared with an atom of -12 isotope taken as .
The mass of molecule molecular mass (in ).
The mass of mole molecules molecular mass (in ).
Example:
The mass of molecules .
The mass of molecules .
(a) For atom atom mole.
For molecule molecule mole.
(b) or of the mass of an atom of -
(c)
(d)
(e) Atomic mass Specific heat (in ) (Dulong-Petit law).
7. Mole Concept:
Moles can be calculated in following manner:
(i) Number of moles of molecules
(ii) Number of moles of atoms
(iii) Number of moles of gases
Mole of any gas occupies a volume of litres at ,
( corresponds to and pressure).
(iv) Number of moles of atoms/molecules/ions
(Avogadro’s constant is equal to ).
8. Empirical and Molecular Formula:
(i) Empirical formula of a compound is the simplest whole number ratio of the atoms of the elements constituting its one molecule. The sum of atomic masses of the atoms representing the empirical formula is called the empirical formula mass.
(ii) Molecular formula of a compound shows the actual number of the atoms of the elements present in its one molecule. The sum of atomic masses of the atoms representing the molecule is called the molecular mass.
(iii) Relationship between the empirical formula and the molecular formula.
Molecular formula = empirical formula, where is a simple whole number having values of , etc.
Also, Molecular formula mass/Empirical formula mass.
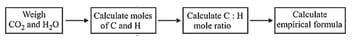
9. Percentage Composition of Compounds:
Percentage Composition of Element:
When a compound is formed from two or more elements, the amount of the element present in a compound is always proportional to its definite mass.
Mass of an element
Example:
Calculate the percentage of both the elements present in water .
Solution:
Atomic mass of .
Atomic mass of .
Molecular mass of .
Mass of Hydrogen
.
Mass of Oxygen
.
10. Chemical Equations and Stoichiometry:
A chemical reaction can be expressed in the form of a chemical equation from which we obtain a large amount of qualitative and quantitative information. Quantitative information is available from the balanced chemical equation available from the stoichiometry of a given reaction. A balanced equation for combustion of methane is given below. Let us consider what information is available from stoichiometry.
| Mole | mole | mole | mole | mole |
| Molecules | molecule | molecule | molecule | molecule |
| Volume at N.T.P. | ||||
| Mass | ||||
| Number of molecules |
The stoichiometry of the reactants in the above reaction is and the stoichiometry of the product is also . Thus, for a general reaction, , the stoichiometries of the reactants and the products are and , respectively.
11. Limiting Reagent:
In the given reaction, if number of quantities (either in /mole/molecules) are present with exact coefficients, the chemical reaction goes to completion without any reactant left unused.
However, if the exact proportion is not present, the one which gets totally consumed is known as the limiting reagent (Limiting reagent decides the product quantity for the given information).
Example:
In the above e.g., moles of react exactly with mole of to give moles of . If the given moles of are moles and that of are , moles will act as a limiting reagent as it is in the minimum amount and the product formation is given w.r.t. , i.e., mole of .
Percentage Yield
Percentage yield of a reaction .
12. Concentration Terms:
(i) Molarity :
It is the number of moles of the solute present per litre of the solution.
millimoles
The molarity changes with the temperature of the solution. Increase in the temperature generally decreases the molarity. It is the most convenient method to express the concentration of the solution. On dilution, the molarity decreases.
(ii) Molality :
Number of moles of the solute present per of the solvent.
It is independent of the temperature since no volume factor is involved in the equation.
(iii) Mole fraction
It is the ratio of the number of moles of one component to the total number of moles present in the solution.
For a system having two components and ,
.
The mole fraction is also independent of the temperature.
(iv) In terms of composition:
by weight .
weight by volume (In case of a solid dissolved in a liquid).
by volume (In case of a liquid dissolved in another liquid).
.
by weight is independent of the temperature, while by volume is temperature dependent.
13. Miscellaneous Conversions:
(i) Mole fraction of the solute into molarity of the solution .
(ii) Molarity into the mole fraction .
(iii) Mole fraction into the molality .
(iv) Molality into the mole fraction .
(v) Molality into the molarity .
(vi) Molarity into the molality .
and are the molar masses of the solvent and the solute, is the density of the solution , Molarity , Molality , Mole fraction of the solvent, Mole fraction of the solute.
