Outline an experimental approach that could demonstrate the complete combustion of hydrocarbons results in the formation of carbon dioxide and water.

Important Questions on Does Organic Chemistry Mean We Can Make Any Substance We Want?
What is an organic substance?
The alkanes discussed in the text are either straight-chain or branched. However, there is a homologous series of alkanes known as cycloalkanes. The structure of cyclohexane () is shown below as a structural formula and a skeletal formula. Cyclohexane is a liquid at room temperature.
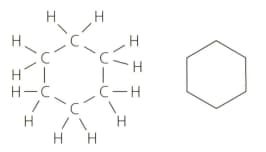
Deduce the general formula for the cycloalkanes and use it to predict the formulas for cycloheptane and cyclooctane.
The alkanes discussed in the text are either straight-chain or branched. However, there is a homologous series of alkanes known as cycloalkanes. The structure of cyclohexane () is shown below as a structural formula and a skeletal formula. Cyclohexane is a liquid at room temperature.
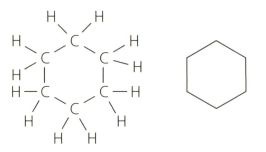
On an industrial scale cyclohexane is prepared by the hydrogenation of benzene (). Formulate an equation summarising this reaction.
Methanol, ethanol, propan--ol and butan--ol are all highly soluble in water, but pentan--ol is far less soluble and decan--ol is almost insoluble at .
A fresh ball of cotton wool is dipped into each of the alcohols listed above and rubbed across a piece of dark paper to produce a series of six ‘wet marks’. Predict, with reasons, the order in which the marks will evaporate to dryness.
