Outline examples of the reaction products of incomplete combustion which may form if oxygen is a limiting reactant.

Important Questions on How Can Our Energy Resources Be Accessed Fairly?
Present each reaction below as a thermochemical equation by including , to indicate whether it is exothermic or endothermic.
Combustion of methanol:
Explain the reaction below as a thermochemical equation by including , to indicate whether it is exothermic or endothermic.
Decomposition of ammonium iodide:
Present each reaction below as a thermochemical equation by including , to indicate whether it is exothermic or endothermic.
Neutralisation of nitric acid by barium hydroxide solution:
Present the reaction below as a thermochemical equation by including , to indicate whether it is exothermic or endothermic.
The reaction between water and calcium oxide forms calcium hydroxide.
Present each reaction below as a thermochemical equation by including , to indicate whether it is exothermic or endothermic.
The burning of potassium in the air to form potassium oxide.
Suggest with reference to the table below:
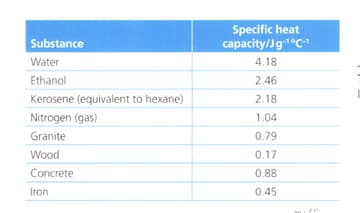
Why water is used in calorimeters?
Suggest with reference to the table below:
Why does ethanol have a higher specific heat capacity than hexane?
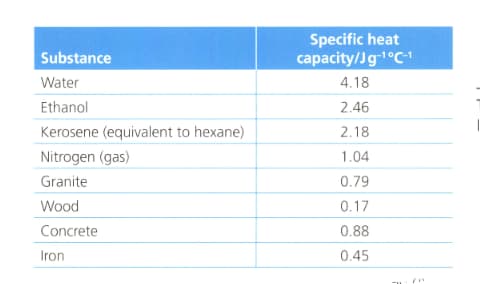
Calculate the amount of thermal energy required to raise the temperature of water in these situations. The specific heat capacity of water is .
of water from
