HARD
JEE Main
IMPORTANT
Earn 100
Which of the following sketches may represent the above equilibrium? Assume equilibrium can be achieved from either side and by taking anyone or more components initially.(Given for the reaction ).
(a)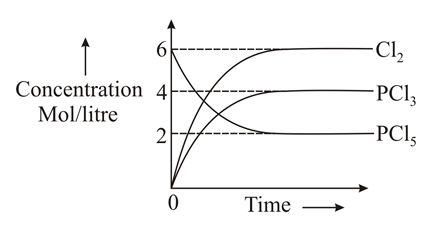

(b)

(c)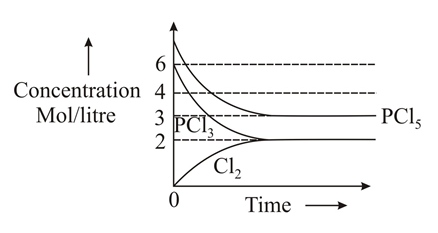

(d)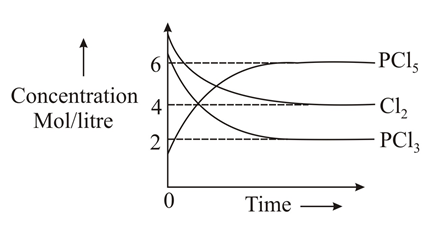

50% studentsanswered this correctly

Important Questions on Chemical Equilibrium
MEDIUM
JEE Main
IMPORTANT
The equilibrium constant of a reaction doubles on increasing the temperature of the reaction from to . Calculate enthalpy change of the reaction, assuming it to be constant in this temperature range.
MEDIUM
JEE Main
IMPORTANT
Sulphide ion in an alkaline solution reacts with solid sulphur to form polysulphide ions having formulacalculate for .
EASY
JEE Main
IMPORTANT
Predict which of the following reaction will have an appreciable concentration of reactants and products:
,
EASY
JEE Main
IMPORTANT
Predict which of the following reaction will have an appreciable concentration of reactants and products:
, .
EASY
JEE Main
IMPORTANT
Predict which of the following reaction will have an appreciable concentration of reactants and products:
,
MEDIUM
JEE Main
IMPORTANT
Determine for the reaction, , from the following data at .
MEDIUM
JEE Main
IMPORTANT
For the reaction, , the dissociation pressure is at and . What will be the dissociation pressure at ?
MEDIUM
JEE Main
IMPORTANT
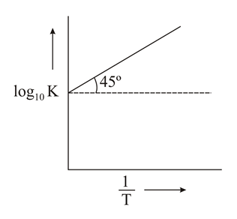
The variation of with is shown in the following graph. for the reaction will be: