MEDIUM
AS and A Level
IMPORTANT
Earn 100
Phenol reacts with bromine water. Give the name of the product, two visual observations and a balanced chemical equation. Then comment on how this shows that phenol is more reactive than benzene.

Important Questions on Benzene and its Compounds
MEDIUM
AS and A Level
IMPORTANT
Contrast the molecules of phenol and benzene to explain why phenol is more reactive than benzene. Your answer must include reference to the model used for the arrangement of electrons.
EASY
AS and A Level
IMPORTANT
Benzene can be nitrated to give nitrobenzene. Name the mechanism for this reaction.
EASY
AS and A Level
IMPORTANT
Benzene can be nitrated to give nitrobenzene. The species attacking benzene in the reaction is . How is generated in the reaction mixture? Name the substances used and give a chemical reaction leading to the formation of .
EASY
AS and A Level
IMPORTANT
Benzene can be nitrated to give nitrobenzene. Give a suitable temperature for this reaction.
HARD
AS and A Level
IMPORTANT
Benzene can be nitrated to give nitrobenzene. Use curly arrows to draw the mechanism of how benzene reacts with to produce nitrobenzene.
MEDIUM
AS and A Level
IMPORTANT
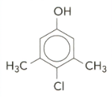
Benzene can be nitrated to give nitrobenzene. The structure of a common household substance is given below:
Give the molecular formula of the compound.
EASY
AS and A Level
IMPORTANT
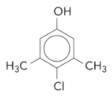
Benzene can be nitrated to give nitrobenzene. The structure of a common household substance is given below:
When bromine water is added to a solution of the compound, it is decolourised. Suggest two structures for the product of the reaction.
HARD
AS and A Level
IMPORTANT
Describe the bonding in benzene. Include a description of the model used for the arrangement of electrons in the molecules.