Predict the major product (s) of the following reactions and explain their formation.

Important Questions on Hydrocarbons
Nucleophiles and electrophiles are reaction intermediates having electron-rich and electron-deficient centres respectively. Hence, they tend to attack electron-deficient and electron-rich centres respectively. Classify the following species as electrophiles and nucleophiles.
Nucleophiles and electrophiles are reaction intermediates having electron-rich and electron-deficient centres respectively. Hence, they tend to attack electron-deficient and electron-rich centres respectively. Classify the following species as electrophiles and nucleophiles.
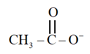
Nucleophiles and electrophiles are reaction intermediates having electron-rich and electron-deficient centres respectively. Hence, they tend to attack electron-deficient and electron-rich centres respectively.
Classify the following species as electrophiles and nucleophiles.
Nucleophiles and electrophiles are reaction intermediates having electron-rich and electron-deficient centres respectively. Hence, they tend to attack electron-deficient and electron-rich centres respectively. Identify the following species is electrophile or nucleophile.
Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Identify the following species is electrophile or nucleophile.
Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Identify the following species is electrophile or nucleophile.
Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Identify the following species is electrophile or nucleophile.
Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Identify the following species is electrophile or nucleophile.
