Pressure volume and temperature for a certain gas are related as , where and are constants. Keeping the pressure constant, what is the work done by the gas as its temperature changes from to ?
Important Questions on Thermodynamics
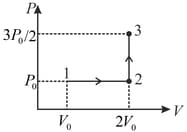
A thermodynamic system undergoes a cyclic process as shown in the diagram. The work done by the system per cycle is
[ universal gas constant ]
An ideal gas is taken reversibly around the cycle as shown on the T (temperature) - S (entropy) diagram
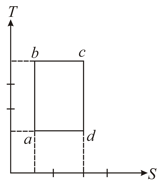
The most appropriate representation of above cycle on a U (internal energy)-V (volume) diagram is
In a thermodynamics process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by where is temperature of the system and is the infinitesimal change in a thermodynamic quantity of the system. For a mole of monatomic ideal gas Here, is gas constant, is volume of gas, and are constants.
The List-I below gives some quantities involved in a process and List-II gives some possible values of these quantities.
| Column - I | Column - II | ||
| (I) | Work done by the system in process | (P) | |
| (II) | Change in internal energy in process | (Q) | |
| (III) | Heat absorbed by the system in process | (R) | |
| (IV) | Heat absorbed by the system in process | (S) | |
| (T) | |||
| (U) |
If the process on one mole of monatomic ideal gas is as shown in the -diagram with the correct match is
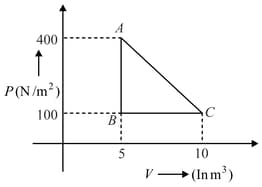
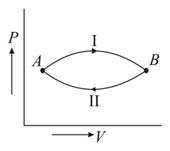
A gas at state changes to state through path and shown in figure. The change in internal energy is and , respectively. Then
The temperature of of an ideal diatomic gas is increased by without changing the pressure of the gas. The molecules in the gas rotate but do not oscillate. If the ratio of change in internal energy of the gas to the amount of workdone by the gas is Then the value of (round off to the nearest integer) is
Given
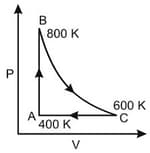
One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement :