HARD
Earn 100
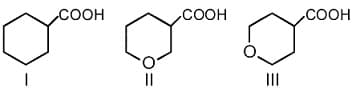
Rank the compounds given below in order of decreasing basicity
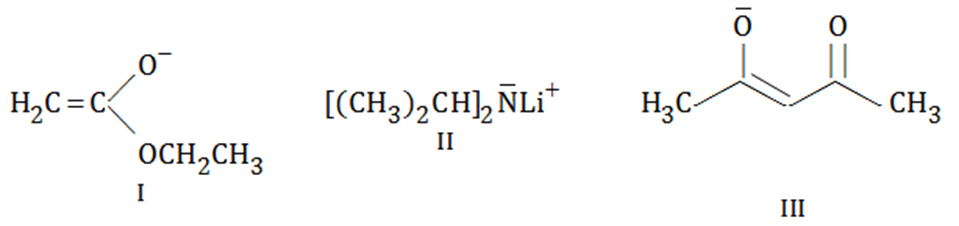
(a)I, II, III
(b)III, II, I
(c)II, III, I
(d)II, I, III
50% studentsanswered this correctly
Important Questions on Some Basic Principles of Organic Chemistry
MEDIUM
The compound that does NOT liberate CO2, on treatment with aqueous sodium bicarbonate solution, is
MEDIUM
The correct statement regarding the basicity of aryl amines is:
EASY
The weakest among the following acids is
EASY
The correct increasing order of the basic strength for the following compounds is:
MEDIUM
The increasing basicity order of the following compounds is:
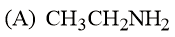
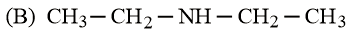
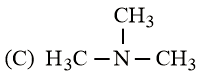
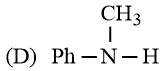
EASY
Among formic acid, acetic acid, propanoic acid and phenol, the strongest acid in water is
MEDIUM
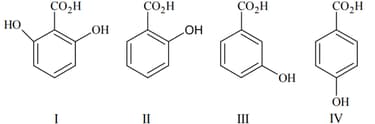
The correct order of acidity of the following compounds is

EASY
The which contribute least to the basicity of the compound is :
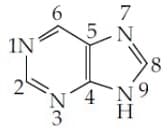
MEDIUM
The correct order for acid strength of compounds and is as follows:
HARD
The correct order of strengths of carboxylic acids is
EASY
Among the following oxoacids, the correct decreasing order of acid strength is :
MEDIUM
The correct order of basicity is
MEDIUM
Considering the basic strength of amines in an aqueous solution, which one has the smallest value?
EASY
The acidity of compounds in water
. Ethanol . Acetic Acid . Phenol . Acetonitrile
follows the order
HARD
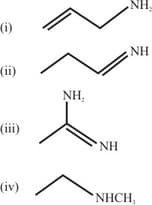
The increasing order of basicity of the following compounds is :
HARD
The increasing order of the of the following compund is:
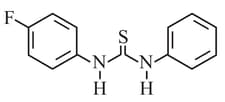
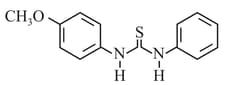
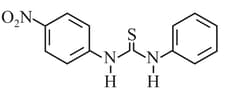
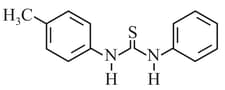
HARD
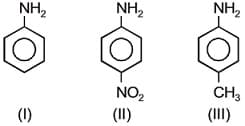
What is the order of basicity among the following compounds?
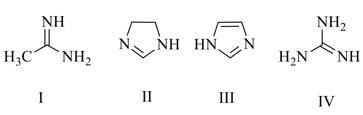
EASY
In case of substituted aniline the group which decreases the basic strength is
MEDIUM
Among the following, which is least acidic?
HARD
The correct order of acidity for the following compounds is :