Read the given statements and select the correct option.
Statement 1: Electrolysis of water is a displacement reaction.
Statement 2: Water gets broken down to form hydrogen and oxygen.
Statement 1: Electrolysis of water is a displacement reaction.
Statement 2: Water gets broken down to form hydrogen and oxygen.

Important Questions on Physical And Chemical Changes
Classify each of the following reactions:
1.
2.
3.
4.
While discussing the topic 'Rusting', Mr. Ankit, a class 7 teacher wrote the following statements on the blackboard. He asked his students to find the incorrect statements.
I. Depositing a layer of tin on iron is called galvanisation.
II. Stainless steel rusts more quickly as it contains carbon and metals like chromium, nickel and manganese.
III. Salty water fastens the process of rust formation.
Select the incorrect statement(s).
A few changes are given below:
A. Making almirah from wood
B. Galvanisation of an iron pot
C. Making an aeroplane from a paper
D. Formation Of acid rain from air pollutants
E. Photosynthesis
F. Breaking of vase
Classify these changes into
I. Irreversible-physical change
II. Chemical change
III. Reversible-physical change
Observe the given figures carefully.
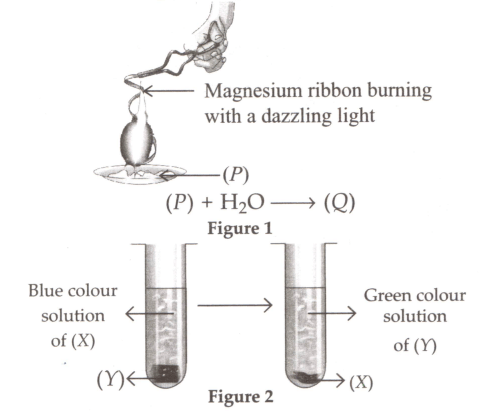
Read the given passage and fill in the blanks by selecting an appropriate option.
Figures 1 and 2 both represent a (i) change. In figure 1, P is (ii) and Q is (iii) . In figure 2, X and Y can be (iv) and (v) respectively.
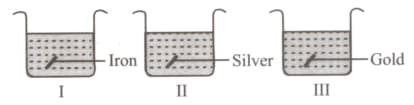
Vishakha took a few wire pieces made up of different metals and placed them in a blue-coloured solution of copper sulphate. What will be the colour of the solutions present in beakers I, II and III after half an hour?
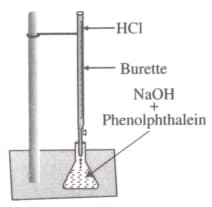
Observe the given figure carefully and select the correct statements.
I. Heat is evolved in this reaction.
II. The colour of the mixture in the conical flask is pink in the beginning.
III. It represents a neutralisation reaction.
IV. No new substance is formed in the reaction.
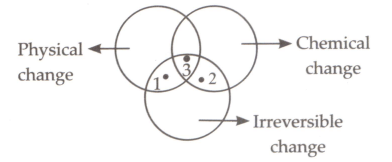
Study the given Venn diagram and identify points 1, 2 and 3.
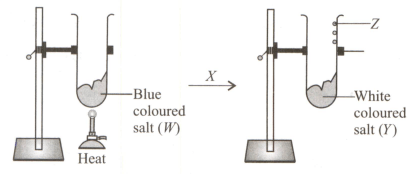
Observe the given change carefully and identify W, X, Y and Z.