HARD
11th CBSE
IMPORTANT
Earn 100
Represent diagrammatically the bond moments and the resultant dipole moment in , and .

Important Questions on Chemical Bonding and Molecular Structure
HARD
11th CBSE
IMPORTANT
Use the molecular orbital energy level diagram to show that would be expected to have a triple bond, , a single bond and , no bond.
HARD
11th CBSE
IMPORTANT
Briefly describe the valence bond theory of covalent bond formation by taking an example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
HARD
11th CBSE
IMPORTANT
Describe hybridisation in the case of and . The axial bonds are longer as compared to equatorial bonds in whereas in both axial bonds and equatorial bonds have the same bond length. Explain.
HARD
11th CBSE
IMPORTANT
Discuss the concept of hybridisaton. What are its different types in a carbon atom.
HARD
11th CBSE
IMPORTANT
What is the type of hybridisation of carbon atoms marked with star.
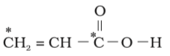
MEDIUM
11th CBSE
IMPORTANT
What is the type of hybridisation of carbon atoms marked with star.
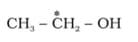
HARD
11th CBSE
IMPORTANT
What is the type of hybridisation of carbon atoms marked with star.
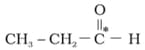
HARD
11th CBSE
IMPORTANT
What is the type of hybridisation of carbon atoms marked with star.
