EASY
NEET
IMPORTANT
Earn 100
Resonance hybrid of nitrate ion is :
(a)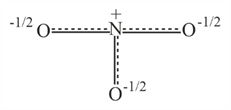

(b)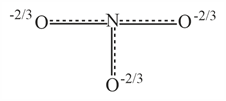

(c)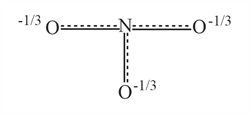

(d)None of the above.
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
HARD
NEET
IMPORTANT
The hybridization of atomic orbitals of nitrogen in - and and :
EASY
NEET
IMPORTANT
Number and type of bonds between two carbon atoms in are:
MEDIUM
NEET
IMPORTANT
In which molecule are all atoms co-planar?
EASY
NEET
IMPORTANT
In which of the following is the angle between the two covalent bonds is the greatest?
EASY
NEET
IMPORTANT
Which of the following molecule does not have a linear arrangement of atoms?
EASY
NEET
IMPORTANT
The molecular orbital configuration of a diatomic molecule is
Its bond order is ____
EASY
NEET
IMPORTANT
The paramagnetic property of the oxygen molecule is due to the presence of unpaired electrons present in
EASY
NEET
IMPORTANT
Which of the following statements is not correct regarding bonding molecular orbitals songer grif
