Rutherford's scattering experiments were done in an evacuated container. Explain why this is necessary.

Important Questions on Atomic Structure and Particle Physics
In Rutherford's experiment, -particles were directed at a thin gold foil. A small fraction of the a-particles were back-scattered through . Describe and explain how the fraction backscattered changes if each of the following changes are (separately) made.
(a) A thicker foil is used.
In Rutherford's experiment, -particles were directed at a thin gold foil. A small fraction of the a-particles were back-scattered through . Describe and explain how the fraction backscattered changes if each of the following changes are (separately) made. Faster -particles are used.
In Rutherford's experiment, -particles were directed at a thin gold foil. A small fraction of the a-particles were back-scattered through . Describe and explain how the fraction backscattered changes if each of the following changes are (separately) made. A silver foil is used - a silver nucleus has less positive charge than a gold nucleus.
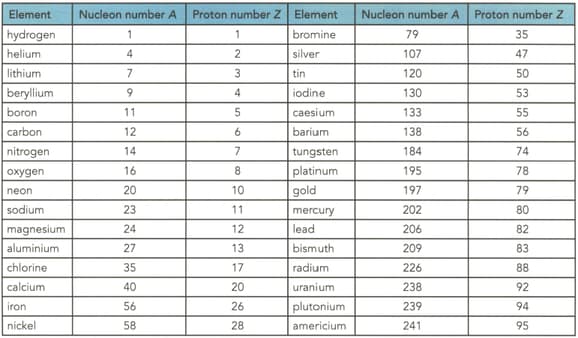
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(a) Nitrogen
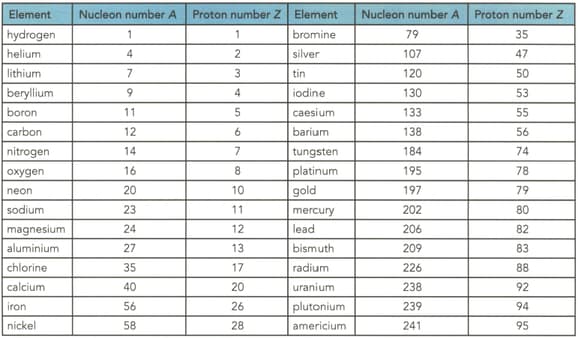
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(b) Bromine
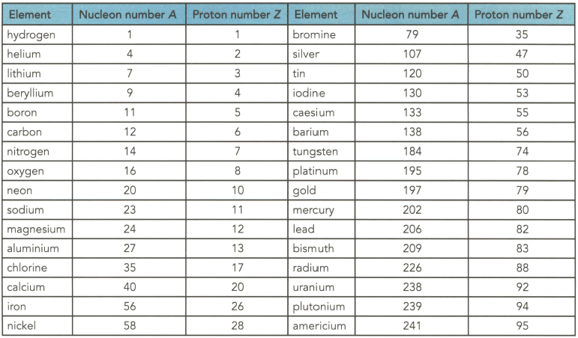
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(c) Silver
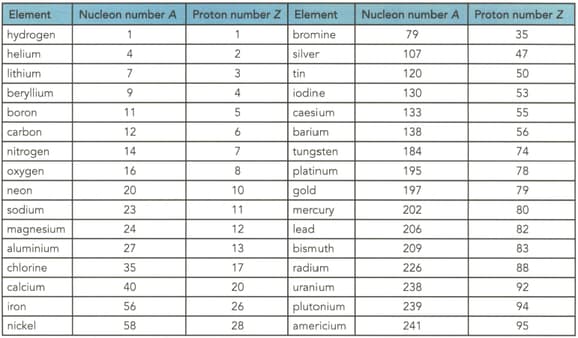
Table shows the proton and nucleon numbers of several nuclei. Determine the number of neutrons in the nuclei of the following elements shown in the table
(d) Gold
