MEDIUM
Earn 100
Select correct statements:
(1) Gases which have high value of van der Waals constant 'a' are easily liquefied.
(2) Easily liquefiable gases are water soluble.
(3) Gases which forms ions in a solvent are soluble in that solvent.
(4) Under same conditions, has low solubility in water than that of
(a)
(b)
(c)
(d)All of the above
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
EASY
HARD
EASY
EASY
EASY
MEDIUM
EASY
HARD
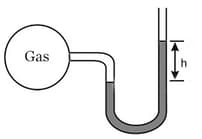
Through a small hole in the bulb, gas is leaked. Assuming, pressure decreases as , if the value of is after . What is the value of ?
HARD
HARD
EASY
EASY
HARD
EASY
EASY
HARD
Give suitable explanation for the following fact about gases.
Gases don’t settle at the bottom of a container.
EASY
EASY
EASY
EASY

