Shape of the compounds and are respectively

Important Points to Remember in Chapter -1 - Chemical Bonding and Molecular Structure from Embibe Experts Gamma Question Bank for Engineering Chemistry Solutions
1. Chemical Bond:
It is the permanent attractive force which holds atoms and Ions together in molecules and compounds.
2. Covalent Bond:
It is formed by sharing of valence electrons between atoms to form molecules e.g., formation of molecule:
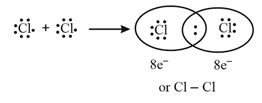
The important conditions:
(i) Each bond is formed because of sharing of an electron pair between the atoms.
(ii) Each combining atom contributes at least one electron to the shared pair.
(iii) The combining atoms attain the outer- shell noble gas configurations because of sharing of electrons.
3. Coordinate Bond (Dative Bond):
The bond formed between two atoms in which contribution of an electron pair is made by one of them while the sharing is done by both.
(i) (ammonium ion)
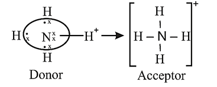
(ii) (ozone)
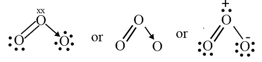
Other examples:
Formal Charge:
Formal charge (F.C.) On an atom in a Lewis structure [Total number of valence electron in the free atom] [ Total number of nonbonding (lone pair) electrons] [ Total number of bonding (shared) electrons]
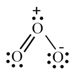
Formal charges help in the selection of the lowest energy structure from several possible Lewis structures for a given species.
4. Limitations of the Octet Rule:
(i) The incomplete octet of the central atom:
, and and .
(ii) Odd-electron molecules:
Nitric oxide, and nitrogen dioxide.
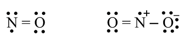
(iii) The expanded octet
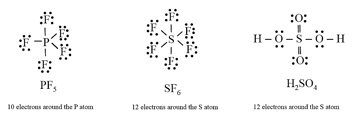
(iv) Other drawbacks of the octet theory:
(a) Some noble gases (for example xenon and krypton) also combine with oxygen and fluorine to form several compounds like etc.
(b) This theory does not account for the shape of molecules.
(c) It does not explain the relative stability of the molecules being totally silent about the energy of a molecule.
5. Ionic or Electrovalent Bond:
The formation of an ionic compound would primarily depend upon:
(i) The ease of formation of the positive and negative ions from the respective neutral atoms.
(ii) The arrangement of the positive and negative ions in the solid, that is the lattice of the crystalline compound.
6. Conditions for the Formation of Ionic Compounds:
(i) Electronegativity difference between two combining elements must be large.
(ii) Ionization enthalpy of electropositive element must be low.
(iii) Negative value of electron gain enthalpy of electronegative element should be high.
(iv) Lattice enthalpy of an ionic solid must be high i.e., large amount of energy should be released during the formation of the ionic salt.
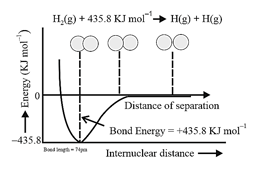
7. Lattice Enthalpy:
The lattice enthalpy of an ionic solid is defined as the energy required to separate one mole of a solid ionic compound into gaseous constituent ions.
Factors affecting lattice energy of an ionic compound:
(i) Lattice energy where Inter-ionic Distance.
(ii) Lattice energy
charge on cation in terms of electronic charge.
charge on anion in terms of electronic charge.
8. Determination of Lattice energy:
Born-Haber Cycle:
It inter-relates the various energy terms involved during formation of an ionic compound. It is a thermochemical cycle based on Hess's law of constant heat summation. It is used to calculate the lattice energy of an ionic compound.
9. Hydration and Hydrolysis of ionic Compounds:
(i) Hydration:
All the simple salts dissolve in water, producing ions, and consequently the solution conducts electricity. Since is very small, it is heavily hydrated. This makes the radius of hydrated ion large and hence, it moves very slowly. In contrast, is the least hydrated because of its bigger size and thus the radius of the ion is smaller than the radius of hydrated and hence hydrated moves faster and conducts electricity more readily.
(ii) Hydrolysis:
Hydrolysis means reaction with water molecules ultimately leading to breaking of O–H bond into and ions. Hydrolysis in covalent compounds takes place generally by two mechanisms:
(a) Formation of acid and bases from Ions derived from weak acid or weak bases: Generally, Ions derived from weak acid and base hydrolyse to give back the corresponding acids or bases.
(b) By mechanism: For example, in Nitrogen trihalides .
10. General properties of Ionic compounds:
(i) Physical state: At room temperature ionic compounds exist either in solid state or in solution phase but not in gaseous state.
(ii) Simple ionic compounds do not show isomerism, but isomorphism is their important characteristic.
e.g.,
(iii) Electrical conductivity and Solubility of ionic compounds:
All ionic solids are good conductors in molten state as well as in their aqueous solutions because their ions are free to move. Moreover, they are Soluble in polar solvents like water which have high dielectric constant.
11. Covalent character in Ionic compounds (Fajan's rule):
Fajan pointed out that greater is the polarization of anion in a molecule, more is covalent character in it.
More distortion of anion, more will be polarisation and the covalent character will also increase.
Fajan gave some rules which govern the covalent character in the ionic compounds, which are as follows:
(i) Size of cation: Size of cation
(ii) Size of anion: Size of anion Polarisation.
(iii) Charge on cation: Charge on cation Polarisation.
(iv) Charge on anion: Charge on anion α Polarisation.
(v) Pseudo inert gas configuration of cation.
12. Valence Bond theory (VBT):
13. Orbital Overlap Concept:
According to the orbital overlap concept, the formation of a covalent bond between two atoms results by pairing of electrons present in the valence shell having opposite spins.
Types of Overlapping and Nature of Covalent Bonds:
The covalent bond may be classified into two types depending upon the types of overlapping:
(i) sigma bond, and (ii) pi bond
(a) Sigma bond: This type of covalent bond is formed by the end to end (head-on) overlap of bonding orbitals along the internuclear axis.
overlapping:
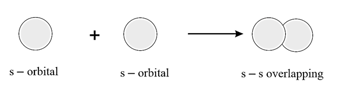
overlapping: It is formed by the overlapping of and orbitals.
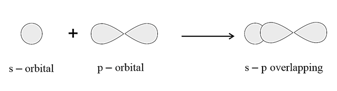
overlapping: This type of overlap takes place between half-filled -orbitals of the two approaching atoms.
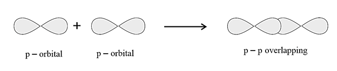
(b) pi bond: In the formation of pi bond the atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the internuclear axis.
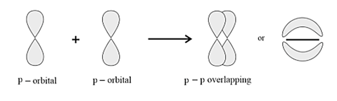
Strength of Sigma and pi Bonds:
In case of sigma bond, the overlapping of orbitals takes place to a larger extent. Hence, it is stronger as compared to the pi bond where the extent of overlapping occurs to a smaller extent.
14. Resonance:
Definition: Resonance may be defined as the phenomenon in which two or more structures involving identical position of atom, can be written for a particular compound.
For example, the ozone, molecule can be equally represented by the structures and shown below:
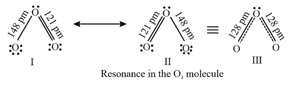
Resonance Hybrid: It is the actual structure of all different possible structures that can be written for the molecule without violating the rules of covalence maxima for the atoms.
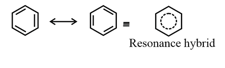
15. Hybridisation:
Salient features of hybridisation:
(i) The number of hybrid orbitals is equal to the number of the atomic orbitals that get hybridised.
(ii) The hybridised orbitals are always equivalent in energy and shape.
(iii) The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
(iv) These hybrid orbitals are directed in space in some preferred direction to have minimum repulsion between electron pairs and thus a stable arrangement is obtained. Therefore, the type of hybridisation indicates the geometry of the molecules.
16. Important conditions for Hybridisation:
(i) The orbitals present in the valence shell of the atom are hybridised.
(ii) The orbitals undergoing hybridisation should have almost equal energy.
(iii) Promotion of electron is not essential condition prior to hybridisation.
(iv) It is the orbital that undergoes hybridization and not the electrons.
Determination of hybridisation of an atom in a molecule or ion:
Steric number rule (given by Gillespie):
Steric No. of an atom number of atoms bonded with that atom number of lone pair(s) left on that atom.
| Steric number | Types of Hybridisation | Geometry |
| Linear | ||
| Trigonal planar | ||
| Tetrahedral | ||
| Trigonal bipyramidal | ||
| Octahedral | ||
| Pentagonal bipyramidal |
Hybridization Involving -orbital:
| Hybridisation | Type of ‘ ’ orbital involved |
|---|---|
| & | |
| , & | |
17. Valence Shell Electron Pair Repulsion Theory (VSEPR):
The main postulates of VSEPR theory are as follows:
(ii) The shape of a molecule depends upon the number of valence shell electron pairs [bonded or non-bonded] around the central atom.
(iii) Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
(iv) These pairs of electrons tend to occupy such positions in space that minimise repulsion and thus maximise distance between them.
(v) The valence shell is taken as a sphere with the electron pairs localising on the spherical surface at maximum distance from one another.
(vi) A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair.
(vii) Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structure.
The repulsive interaction of electron pairs decreases in the order:
18. Dipole Moment:
Dipole moment Magnitude of charge distance of separation
Dipole moment is usually expressed in Debye units . The conversion factors are
, where is coulomb and is meter.
.
For example, the dipole moment of HF may be represented as
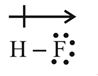
The shift electron density is represented by crossed arrow  about the Lewis structure to indicate the direction of the shift.
about the Lewis structure to indicate the direction of the shift.
A molecule will have a dipole moment if the summation of all the individual moment vector is non- zero.
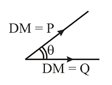
, where is resultant dipole moment.
19. Hydrogen Bond:
Conditions required for -bond:
(i) Molecule should have more electronegative atom linked to -atom.
(ii) Size of electronegative atom should be smaller.
(iii) A lone pair should be present on electronegative atom.
Order of -bond strength

20. Types of -bonds:
Intramolecular -Bonding:
It is formed when hydrogen atom is present in between the two highly electronegative atoms within the same molecule.
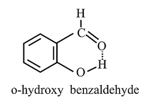
It has lower boiling point (i.e., more volatile) than its para-derivative
Necessary conditions for the formation of intramolecular hydrogen-bonding:
(i) the ring formed as a result of hydrogen bonding should be planar.
(ii) a - or - membered ring should be formed.
(iii) interacting atoms should be placed in such a way that there is minimum strain during the ring closure.
Intermolecular -Bonding:
It is formed between two different molecules of the same or different compounds,
(i) In water molecules:
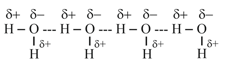
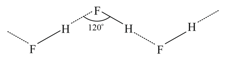
(ii) The hydrogen bonds in link the F atom of one molecule with the H-atom of another molecule, thus forming a zig-zag chain in both the solid and liquid state.
21. Molecular Orbital Theory (MOT):
Developed by F. Hund and R.S. Mulliken in .
(i) Molecular orbitals are formed by the combination of atomic orbitals of comparable energies and proper symmetry.
(ii) An electron in an atomic orbital is influenced by one nucleus, while in a molecular orbital it is influenced by two or more nuclei depending upon the number of the atoms in the molecule. Thus, an atomic orbital is monocentric while a molecular orbital is polycentric.
(iii) The number of molecular orbitals formed is equal to the number of combining atomic orbitals. When two atomic orbitals combine, two molecular orbitals called bonding molecular orbital and anti-bonding molecular orbital are formed.
(iv) The molecular orbitals like the atomic orbitals are filled in accordance with the Aufbau principle obeying the Pauli Exclusion principle and the Hund’s Rule of Maximum Multiplicity. But the filling order of these molecular orbitals is always experimentally decided, there is no rule like rule in case of atomic orbitals.
22. Conditions for the combination of atomic orbitals:
(i) The combining atomic orbitals must have the same or nearly the same energy.
(ii) The combining atomic orbitals must have the same symmetry about the molecular axis.
(iii) The combining atomic orbitals must overlap to the maximum extent.
23. Energy level diagram for Molecular orbitals:
(i) The increasing order of energies of various molecular orbitals for and is given below:
(ii) The important characteristic feature of this order is that the energy of molecular orbital is higher than that of and molecular orbitals.
(iii) Bond order (B.O.)
(iv) A positive bond order (i.e., ) means a stable molecule while a negative (i.e., ) or zero (i.e., ) bond order means an unstable molecule.
(v) Nature of the bond: Integral bond order values of or correspond to single, double, or triple bonds, respectively.
(vi) Bond-length: The bond order between two atoms in a molecule may be taken as an approximate measure of the bond length. The bond length decreases as bond order increases.
(vii) Magnetic nature: If all the molecular orbitals in a molecule are doubly occupied, the substance is diamagnetic (repelled by magnetic field) e.g., molecule.
24. Intermolecular forces (Van der Waal’s Forces):
Intermolecular attractions hold two or more molecules together. These are weakest chemical forces and can be of following types.
(i) Ion-dipole attraction.
(ii) Dipole-dipole attraction.
(iii) Ion-induced dipole attraction.
(iv) Dipole-induced dipole attraction.
(v) Instantaneous dipole- Instantaneous induced dipole attraction: (Dispersion force or London forces)
(vi) Strength of Van der Waal force molecular mass.
(vii) van der Waal's force boiling point.
Metallic bond:
Two models are considered to explain metallic bonding:
(i) Electron-sea model.
(ii) Band model.
25. Some special Bonding situations:
(i) Electron deficient bonding:
There are many compounds in which some electron deficient bonds are present apart from normal covalent bonds or coordinate bonds. These electron deficient bonds have lower number of electrons than the expected such as three centre-two electron bonds present in diborane and bridging metal carbonyls.
(ii) Back Bonding:
Back bonding generally takes place when out of two bonded atoms one of the atoms has vacant orbitals (generally this atom is from the second or third period) and the other bonded atom is having some non-bonded electron pair (generally this atom is from the second period). Back bonding increases the bond strength and decreases the bond length. For example, in . The overlapping has been shown in one of the canonical forms.
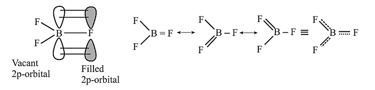
The extent of back bonding in boron trihalides:
