EASY
Earn 100
Sodium hydroxide solution can be used to identify zinc ions.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Inorganic Qualitative Analysis
MEDIUM
MEDIUM
For the following Assertion and Reason, the correct option is
Assertion (A) : When (II) and sulphide ions are mixed, they react together extremely quickly to give a solid.
Reason (R) : The equilibrium constant of is high because the solubility product is low.
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
| List - I (Metal ion) | List - II (Group in Qualitative analysis) | ||
| (a) | (i) | Group - III | |
| (b) | (ii) | Group - IIA | |
| (c) | (iii) | Group - IV | |
| (d) | (iv) | Group - IIB |
EASY
EASY
MEDIUM
MEDIUM
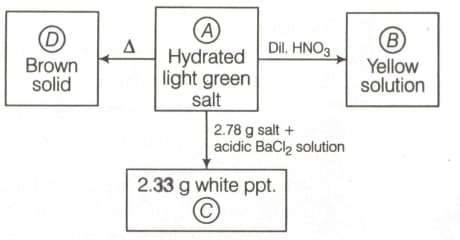
What are ?
HARD
A mixture of two salts is used to prepare a solution which gives the following results:
The correct option for the salt mixture is(are)
MEDIUM
HARD
HARD
HARD

