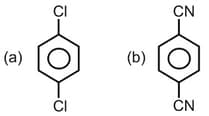
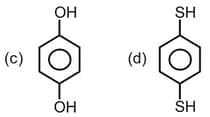
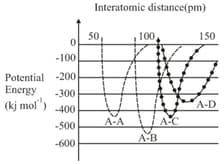
Important Questions on Chemical Bonding and Molecular Structure The bond angle H - O - O H 2 O 2 Amongst LiCl, RbCl, BeCl2 and MgCl2 the compounds with the greatest and the least ionic character, respectively are : Which of the following molecules has the maximum dipole moment? The dipole moments of C C l 4 , C H C l 3 C H 4 Which compounds exhibits maximum dipole moment among the following? The number of bonds between sulphur and oxygen atoms in S 2 O 8 2 - For which of the following molecule significant μ ≠ 0 ? The correct order of C - O Maximum bond angle at nitrogen is present in which of the following? Which of the following molecules has no dipole moment? If enthalpy of atomization for B r 2 ( l ) x k J / m o l B r 2 y k J / m o l The decreasing order of bond angles in BF 3 , NH 3 , PF 3 I 3 - The number of π σ Arrange the following compounds in increasing order of C - O H p Which of the following possess net dipole moment? The compound that has the largest H – M – H ( M = N , O , S , C ) The intermolecular potential energy for the moleculesA B C D Among the following molecules, the one with the largest bond angle at the central atom is Molecule AB has a bond length of 1.617 Å ( e 0 = 4.802 × 10 - 10 e s u ) Which of the alkaline earth metal halides given below is essentially covalent in nature?