EASY
Earn 100
Statement = The following graph illustrates Charles law
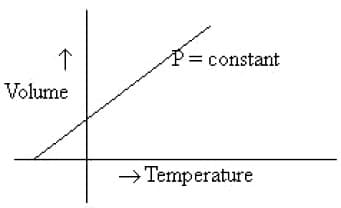
Explanation = Charles law states that at constant pressure, volume of a gas is directly proportional to its absolute temperature
(a)S is correct but E is wrong
(b)S is wrong but E is correct
(c)Both S and E are correct and E is correct explanation of S
(d)Both S and E are correct and E is not correct explanation of S
33.33% studentsanswered this correctly
Important Questions on States of matter: Gaseous and liquid states
MEDIUM
MEDIUM
[Gas constant, ]
EASY
EASY
MEDIUM
EASY
EASY
HARD
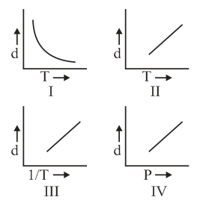
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
EASY
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
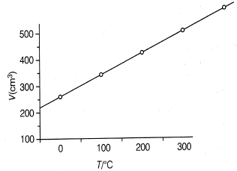
The volume of the gas at is larger than that at by a factor of
MEDIUM
MEDIUM
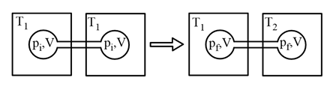
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
MEDIUM
HARD
EASY
HARD
EASY
EASY
MEDIUM
EASY

