EASY
NEET
IMPORTANT
Earn 100
Statement-: The volatility of is more than at a given temperature.
Statement-: The extent of -bonding in is more than ethanol .
(a)Statement-is True, Statement- is True; Statement- is a correct explanation for Statement-.
(b)Statement-.is True, Statement- is True; Statement-. is NOT a correct explanation for Statement- .
(c)Statement- is True, Statement- is False.
(d)Statement- is False, Statement- is True.
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
Which cationic species has more polarising power-
EASY
NEET
IMPORTANT
Which option is correct for the following order .
EASY
NEET
IMPORTANT
Choose incorrect option:
EASY
NEET
IMPORTANT
Among , the compounds with the greatest and the least ionic character, respectively are
EASY
NEET
IMPORTANT
The correct order of increasing covalent character is:
EASY
NEET
IMPORTANT
The volatility of HF is low because of:
EASY
NEET
IMPORTANT
The type of molecular force of ättraction present in the following compound is
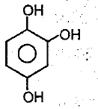
EASY
NEET
IMPORTANT
Which of the following substances does not exhibit H-bonding with water?
