Statement I : Liquid-liquid junction potential can be eliminated by putting a salt bridge of in electrochemical cell.
Statement II : The function of salt bridge is to remove liquid-liquid junction potential because the salt used has same speed of cations and anions.
Important Questions on Electrochemistry
Depict the galvanic cell in which the reaction takes place. Further show, which of the electrode is negatively charged.
.
At , the of the galvanic cell mentioned below is
The following reaction takes place at in an electrochemical cell involving two metals and ,
with and in the respective half-cells, the cell EMF is . The equilibrium constant of the reaction is closest to;
,
Represent a cell consisting of | half cell and | half cell and write the cell reaction.
The reaction occurs in which of the given galvanic cell?
Some materials are given below:
From the given materials, choose the appropriate ones to construct a galvanic cell and draw the diagram of the cell.
(Order of reactivity: )
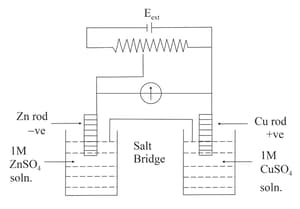
Identify the incorrect statement from the options below for the above cell:
For the cell , when the concentration of is times the concentration of , the expression for is
Faraday's constant, universal gas constant, temperature,

