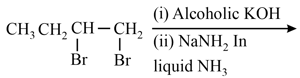MEDIUM
Earn 100
Statement I : The 3-chloro-3, 4-dimethyl hex-1-ene undergo reaction with base to give butadiene.
Statement II : This is due to Sytzeff rule which states that more substituted product will be the more stable product.
(a)Both Statement I and Statement II are true and the Statement II is the correct explanation of the Statement I.
(b)Both Statement I and Statement II are true and the Statement II is not the correct explanation of the Statement I.
(c)Statement I is false but Statement II is true.
(d)Both Statement I and Statement II are false.
50% studentsanswered this correctly
Important Questions on Hydrocarbons
MEDIUM
MEDIUM
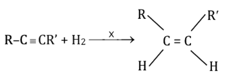
Consider the following reactions:
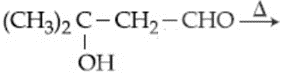
Which of the reaction(s) will not produce Saytzeff product?
HARD
The major product in the following sequence of reaction is :
EASY
HARD
EASY
MEDIUM
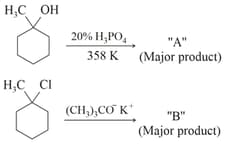
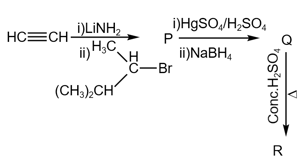
The product "" and "" formed in above reactions are
EASY
HARD
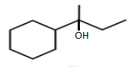 ?
?EASY
EASY
EASY
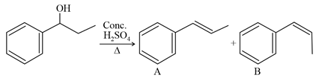
consider the above reaction, and choose the correct statement:
HARD
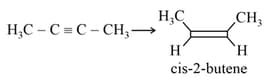
EASY
HARD